Diabetes and periodontitis: the role of a high-glucose microenvironment in periodontal tissue cells and corresponding therapeutic strategies
- PMID: 40660319
- PMCID: PMC12261607
- DOI: 10.1186/s13287-025-04441-z
Diabetes and periodontitis: the role of a high-glucose microenvironment in periodontal tissue cells and corresponding therapeutic strategies
Abstract
Background: Periodontitis is a chronic inflammatory disease that leads to the destruction of periodontal tissues and diabetes is a metabolic disease characterized by hyperglycemia. Both of these diseases affect many people worldwide. Epidemiological data have revealed a close relationship between periodontitis and diabetes. In particular, the high-glucose microenvironment plays an important role in the relationship between these two chronic inflammatory diseases, which makes it difficult to mitigate the progression of periodontitis and restore periodontal tissue in diabetic patients. Anti-inflammatory and regenerative processes are of fundamental importance for periodontal treatment and are mediated by diverse cell populations, including macrophages, T cells, neutrophils, stem cells, and fibroblasts that reside within periodontal tissues.
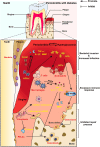
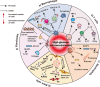
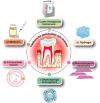
Main body: This review summarizes the interaction between diabetes mellitus and periodontitis, and illustrates that the high-glucose microenvironment aggravates periodontal homeostasis. Furthermore, the mechanism by which the high-glucose microenvironment regulates the involvement of various cells in the destruction of periodontal tissue, leading to the significant inhibition of tissue regeneration and recovery, is discussed. On this basis, the current therapeutic strategies that can be used to target cells are summarized to improve the regeneration and repair processes in the high-glucose microenvironment.
Conclusion: In this review, we assess how metabolic dysregulation mediated by a high-glucose microenvironment exacerbates inflammatory damage and inhibits tissue repair. A deeper understanding of the effects of a high-glucose microenvironment on periodontal tissue cells is essential for developing new therapeutic strategies to restore the structure and function of periodontal tissue.
Keywords: High-glucose microenvironment; Immune cells; Periodontitis; Stem cells; Therapeutic strategies.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures

Similar articles
-
Treatment of periodontitis for glycaemic control in people with diabetes mellitus.Cochrane Database Syst Rev. 2022 Apr 14;4(4):CD004714. doi: 10.1002/14651858.CD004714.pub4. Cochrane Database Syst Rev. 2022. PMID: 35420698 Free PMC article.
-
Treatment of periodontal disease for glycaemic control in people with diabetes mellitus.Cochrane Database Syst Rev. 2015 Nov 6;2015(11):CD004714. doi: 10.1002/14651858.CD004714.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2022 Apr 14;4:CD004714. doi: 10.1002/14651858.CD004714.pub4. PMID: 26545069 Free PMC article. Updated.
-
Adjunctive antimicrobial photodynamic therapy for treating periodontal and peri-implant diseases.Cochrane Database Syst Rev. 2024 Jul 12;7(7):CD011778. doi: 10.1002/14651858.CD011778.pub2. Cochrane Database Syst Rev. 2024. PMID: 38994711 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Should We Be Concerned about the Association of Diabetes Mellitus and Periodontal Disease in the Risk of Infection by SARS-CoV-2? A Systematic Review and Hypothesis.Medicina (Kaunas). 2021 May 13;57(5):493. doi: 10.3390/medicina57050493. Medicina (Kaunas). 2021. PMID: 34068221 Free PMC article.
References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and National incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1789–858. - PMC - PubMed
-
- Luo LS, Luan HH, Wu L, et al. Secular trends in severe periodontitis incidence, prevalence and disability-adjusted life years in five Asian countries: A comparative study from 1990 to 2017. J Clin Periodontol. 2021;48(5):627–37. - PubMed
-
- Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a Tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7(12):738–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

