Mortality and antibiotic timing in deep learning-derived surviving sepsis campaign risk groups: a multicenter study
- PMID: 40660326
- PMCID: PMC12257722
- DOI: 10.1186/s13054-025-05493-6
Mortality and antibiotic timing in deep learning-derived surviving sepsis campaign risk groups: a multicenter study
Abstract
Background: The current Surviving Sepsis Campaign (SSC) guidelines provide recommendations on timing of administering antibiotics in sepsis patients based on probability of sepsis and presence of shock. However, there have been minimal efforts to stratify patients objectively into these groups and describe patient outcomes as a function of antibiotic timing recommendations based on risk stratification using this approach.
Methods: We conducted an observational cohort study using prospectively applied patient data from two large health systems using patient encounters between 2016 and 2024. At the time of clinical suspicion of sepsis, two deep learning (DL) models were used to stratify patients objectively into groups analogous to the SSC risk groups, based on a patient's likelihood of having sepsis and likelihood of developing shock. These risk groups were: (1) shock likely to develop and sepsis probable, (2) shock likely to develop and sepsis possible, (3) shock unlikely to develop and sepsis probable, and (4) shock unlikely to develop and sepsis possible. The primary outcome was short-term mortality, a composite of in-hospital mortality and transition to hospice care, across each risk group.
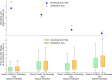
Results: We identified 34,087 adult patients with potential sepsis. At the development site, risk group mortality rates (%) and median time to antibiotics [IQR] were as follows: (1) 23.2%, 1.7 [1.0-3.1] hours; (2) 17.7%, 3.0 [1.7-6.2] hours; (3) 5.0%, 2.8 [1.5-5.1] hours; and (4) 1.9%, 4.6 [2.7-8.0] hours. Results from the validation site were similar. Mortality rates were similar for patients with possible sepsis unlikely to develop shock regardless of antibiotic administration within 1, 3 or more hours from triage. For patients with probable sepsis at the development site, regardless of risk of shock, mortality was significantly lower if antibiotics were administered within the first hour from triage.
Conclusions: Our data suggest that patients who are at low risk of developing shock and possible sepsis had similar rates of mortality in the 1-hour vs. > 1-hour and 3-hour vs. > 3-hour time to antibiotic administration groups. Thus, a more lenient time to antibiotic administration could allow for more detailed evaluations and judicious administration of antibiotics without impacting patient mortality. Patients with probable sepsis had lower mortality if antibiotics were administered within 1 h from triage, regardless of risk of shock. Additional prospective studies are required to validate these findings and guide optimal antibiotic timing in patients with suspected sepsis.
Keywords: Artificial intelligence; Clinical decision support; Septic shock; Sepsis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Institutional review board approval with waiver of informed consent was obtained. Consent for publication: Not applicable. Competing interests: S.N., A. B., and A.M. are cofounders, advisors, and hold equity in Clairyon Inc. (formerly Healcisio Inc.), a medical software startup. The terms of this arrangement have been reviewed and approved by the UC San Diego in accordance with its conflict-of-interest policies. S.N. and A.M. report income from Powell Mansfield, a startup focused on diagnosis of sleep disordered breathing. A.M. additionally reports income related to medical education from Livanova, Eli Lilly, and Zoll. The remaining authors declare no competing interests.
Figures



Update of
-
Mortality and Antibiotic Timing in Deep Learning-Derived Surviving Sepsis Campaign Risk Groups: A Multicenter Study.Res Sq [Preprint]. 2025 Apr 1:rs.3.rs-6123541. doi: 10.21203/rs.3.rs-6123541/v1. Res Sq. 2025. Update in: Crit Care. 2025 Jul 14;29(1):302. doi: 10.1186/s13054-025-05493-6. PMID: 40235491 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
- R35 GM143121/GM/NIGMS NIH HHS/United States
- K23 GM146092/GM/NIGMS NIH HHS/United States
- T15 LM011271/LM/NLM NIH HHS/United States
- R01LM013998/U.S. National Library of Medicine,United States
- R35GM143121/National Institute of General Medical Sciences,United States
- K23GM146092/National Institutes of Health,United States
- M25PR9018/University of California, Multicampus Research Programs and Initiatives
- R42 AI177108/AI/NIAID NIH HHS/United States
- R01 LM013998/LM/NLM NIH HHS/United States
- R42AI177108/National Institute of Allergy and Infectious Diseases
- 2405974/National Science Foundation
LinkOut - more resources
Full Text Sources
Medical

