Spectrophotometric approach for deconvolving overlapped spectra of antihypertensive drug mixtures using UV detection: an eco-friendly method
- PMID: 40660360
- PMCID: PMC12257698
- DOI: 10.1186/s13065-025-01565-4
Spectrophotometric approach for deconvolving overlapped spectra of antihypertensive drug mixtures using UV detection: an eco-friendly method
Abstract
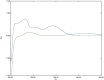
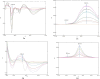
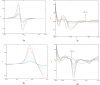
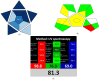
This study presented four simple, environmentally friendly, chemometric methods for the determination of Amlodipine besylate and Telmisartan, When analyzed together, these drugs exhibit spectral overlapping; therefore, by adopting spectral manipulation, drugs are quantified simultaneously. The methods are namely the first derivative spectrophotometric method, the ratio difference method, the first derivative ratio method and the amplitude factor method. The methods showed detection limits ranging from 0.1211 to 0.4304 µg/mL and 0.0773 to 0.5640 µg/mL for Amlodipine besylate and Telmisartan, respectively, with good adherence to International Council for Harmonization standards. The methods were compared with the reported HPLC method using the student t-test and F-test, where the results showed no significant difference. For this method, a green solvent such as propylene glycol is selected through the Green solvent selection tool with a greenness score of 7.8 and the sustainability of the solvent is evaluated using spider diagram. Methods were assessed for their eco-friendliness and sustainability using novel tools such as Blue Applicability Grade Index, Green Analytical Procedure Index and RGB model with respect to White and Green analytical chemistry principles. Using Greenness tools for this UV-spectroscopy method improves both safety and effectiveness compared to expensive and laborious HPLC techniques. Hence, this study suggests employing easy and renewable UV spectrophotometric technologies for traditional analysis.
Keywords: Amlodipine besylate; Spectral overlap; Sustainable method; Telmisartan; UV spectroscopy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Kario K, Okura A, Hoshide S, Mogi M. The WHO global report 2023 on hypertension warning the emerging hypertension burden in Globe and its treatment strategy. Hypertens Res. 2024;47:1099–102. - PubMed
-
- Kjeldsen SE. Hypertension and cardiovascular risk: general aspects. Pharmacol Res. 2018;129:95–9. - PubMed
-
- Ihm S-H, Jeon H-K, Cha T-J, Hong T-J, Kim S-H, Lee N-H, et al. Efficacy and safety of two fixed-dose combinations of S-amlodipine and Telmisartan (CKD-828) versus S-amlodipine monotherapy in patients with hypertension inadequately controlled using S-amlodipine monotherapy: an 8-week, multicenter, randomized, double-blind, phase III clinical study. Drug Des Devel Ther. 2016;10:3817–26. - PMC - PubMed
-
- Laurent S. Antihypertensive drugs. Pharmacol Res. 2017;124:116–25. - PubMed
LinkOut - more resources
Full Text Sources
