Optimization of pathogen detection in abscess specimens: a 6-year retrospective study
- PMID: 40660369
- PMCID: PMC12261717
- DOI: 10.1186/s40001-025-02896-7
Optimization of pathogen detection in abscess specimens: a 6-year retrospective study
Abstract
Background: This study aimed to evaluate the impact of optimized diagnostic protocols on pathogen detection rates in abscess specimens.
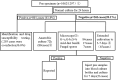
Methods: Our retrospective study analyzed 1,297 abscess specimens collected between 2018 and 2024 using an enhanced diagnostic protocol combining four key methodologies: routine aerobic/anaerobic culture, gram-stain microscopy, acid-fast bacilli staining, and blood culture bottle enrichment techniques.
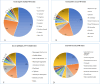
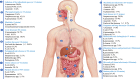
Results: The implementation of optimized diagnostic protocols significantly enhanced pathogen detection efficacy (P < 0.001, χ = 9.663), achieving an overall positivity rate of 81.9% (1,062/1,297)-a 20.1 percentage point improvement over conventional methods. Among culture-positive specimens, polymicrobial infections were identified in 27.6% of cases (293/1,062). A total of 1,651 microbial isolates were recovered, dominated by gram-negative bacteria (50.6%, 836/1,651) with Escherichia coli (55.0%), Klebsiella pneumoniae (23.0%), and Acinetobacter baumannii (4.0%) as predominant species. Gram-positive cocci accounted for 33.7% (557/1,651), primarily Streptococcus spp. (45.0%), Staphylococcus aureus (19.0%), and Enterococcus faecium (6.0%). Enhanced methodology detected 261 additional pathogens (20.1% of total yield), including anaerobes (33.7%), smear-positive organisms (32.2%), acid-fast bacilli (6.9%), and Brucella melitensis (1.5%). Anatomic distribution analysis revealed perianal abscesses (358 cases, 407 isolates) predominantly associated with E. coli (51.8%), K. pneumoniae (14.7%), and Streptococcus spp. (15.7%), followed by maxillofacial infections (244 cases, 297 isolates; 18.0%). Other significant sites included abdominal abscesses/peritonitis (17.0%), hepatic abscesses (5.2%), and Periappendicular abscess (5.5%).
Conclusions: Systematic optimization of diagnostic protocols significantly enhanced pathogen detection in abscess specimens, demonstrating substantial clinical utility for infectious disease management. These findings support the adoption of comprehensive, standardized approaches for abscess specimen processing.
Keywords: Abscess specimen; Anaerobic bacteria; Epidemiology; Optimized standardized operating procedures; Pathogen.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the 940th Hospital Committee for Medical and Health Research Ethics. The Ethics Committee waived the need for informed consent, as this was an observational study, the treatment of the patients was standard, and no samples were taken for the study. Consent for publication: All authors agree to publish articles in the journal. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
A single-center retrospective study of pathogen distribution and antibiotic resistance of bloodstream infections in emergency department.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Nov 28;49(11):1799-1807. doi: 10.11817/j.issn.1672-7347.2024.240333. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 40177763 Free PMC article. Chinese, English.
-
Microbial isolates and resistance profiles in cerebrospinal fluid cultures: a five-year experience at a tertiary center.Future Microbiol. 2025 Jul;20(10):669-680. doi: 10.1080/17460913.2025.2520666. Epub 2025 Jun 18. Future Microbiol. 2025. PMID: 40528708
-
Respiratory bacterial and viral pathogen spectrum among influenza-positive and influenza-negative patients.BMC Infect Dis. 2025 Jul 1;25(1):866. doi: 10.1186/s12879-025-11232-7. BMC Infect Dis. 2025. PMID: 40597844 Free PMC article.
-
Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults.Cochrane Database Syst Rev. 2013 Jan 31;(1):CD009593. doi: 10.1002/14651858.CD009593.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2014 Jan 21;(1):CD009593. doi: 10.1002/14651858.CD009593.pub3. PMID: 23440842 Free PMC article. Updated.
-
Xpert MTB/RIF Ultra assay for tuberculosis disease and rifampicin resistance in children.Cochrane Database Syst Rev. 2022 Sep 6;9(9):CD013359. doi: 10.1002/14651858.CD013359.pub3. Cochrane Database Syst Rev. 2022. PMID: 36065889 Free PMC article.
References
-
- Miller JM, Binnicker MJ, Campbell S, et al. Guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2024 update by the infectious diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2024. 10.1093/cid/ciae104. - PubMed
-
- Rupnik M, Kovács KL, Nagaraja TG, Allen-Vercoe E. Anaerobes in the microbiome. Anaerobe. 2021;68: 102362. 10.1016/j.anaerobe.2021.102362. - PubMed
-
- Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gonzalez MD, Harrington A, Jerris RC, Kehl SC, Leal SM Jr, Patel R, Pritt BS, Richter SS, Robinson-Dunn B, Snyder JW, Telford S 3rd, Theel ES, Thomson RB Jr, Weinstein MP, Yao JD. Guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2024 Update by the Infectious Diseases Society of America (IDSA) and the American Society for Microbiology (ASM). Clin Infect Dis. 2024. 10.1093/cid/ciae104. - PubMed
-
- Jorgensen JH, Pfaller MA. Manual of Clinical Microbiology. 12th ed. Washington, DC: ASM Press; 2019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

