T cells in cancer: mechanistic insights and therapeutic advances
- PMID: 40660400
- PMCID: PMC12261716
- DOI: 10.1186/s40364-025-00807-w
T cells in cancer: mechanistic insights and therapeutic advances
Abstract
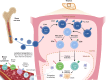
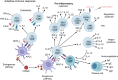
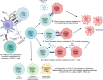
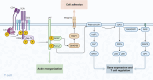
T cells are central players in the fight against cancer, capable of recognizing and destroying tumor cells. However, tumors often find ways to evade this immune response, creating challenges for effective treatment. In this review, we explore how different T cell subsets—including cytotoxic T cells, helper T cells, regulatory T cells, and unconventional T cells—contribute to tumor progression or suppression. We also delve into key mechanisms, such as immune checkpoints and metabolic pathways, that shape T cell behavior in the tumor microenvironment. Advances in cancer immunotherapy, including immune checkpoint inhibitors (ICIs), T cell engagers (TCEs), adoptive T cell therapies (ACTs), chimeric antigen receptor (CAR) T cell therapies, and cancer vaccines, have transformed cancer treatment and provided new hope for patients. However, challenges such as treatment resistance, limited efficacy in solid tumors, and therapy-associated toxicities remain significant barriers to broader clinical success. We discuss innovative strategies to tackle these challenges, including combination therapies and next-generation T cell engineering approaches. By connecting the biology of T cells with cutting-edge therapeutic advances, this review aims to inspire progress in the development of more effective and personalized cancer treatments.
Keywords: Adoptive T cell therapy; Cancer immunotherapy; Precision treatment; T cells; Tumor microenvironment.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
A bibliometric analysis of challenges and advancements in the integrated application of nanoparticles and chimeric antigen receptor T cell therapy.Hum Vaccin Immunother. 2025 Dec;21(1):2518634. doi: 10.1080/21645515.2025.2518634. Epub 2025 Jun 17. Hum Vaccin Immunother. 2025. PMID: 40527861 Free PMC article.
-
T cell subsets in cervical cancer tumor microenvironment: advances and therapeutic opportunities.Front Immunol. 2025 Jun 5;16:1612032. doi: 10.3389/fimmu.2025.1612032. eCollection 2025. Front Immunol. 2025. PMID: 40539072 Free PMC article. Review.
-
Immuno-Oncology at the Crossroads: Confronting Challenges in the Quest for Effective Cancer Therapies.Int J Mol Sci. 2025 Jun 26;26(13):6177. doi: 10.3390/ijms26136177. Int J Mol Sci. 2025. PMID: 40649958 Free PMC article. Review.
-
CAR-Based Cell Therapy in Head and Neck Cancer: A Comprehensive Review on Clinical Applicability.Cancers (Basel). 2025 Jul 1;17(13):2215. doi: 10.3390/cancers17132215. Cancers (Basel). 2025. PMID: 40647513 Free PMC article. Review.
-
Innovative pan-tumor target strategy for CAR-T therapy: cancer-specific exons as novel targets for pediatric solid and brain tumors.J Transl Med. 2024 Nov 12;22(1):1019. doi: 10.1186/s12967-024-05861-w. J Transl Med. 2024. PMID: 39533264 Free PMC article.
References
-
- Jiang S, Yan W. T-cell immunometabolism against cancer. Cancer Lett. 2016;382(2):255–8. - PubMed
-
- Slaney CY, Kershaw MH, Darcy PK. Trafficking of T cells into tumors. Cancer Res. 2014;74(24):7168–74. - PubMed
-
- Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–71. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

