Whey Protein Mitigates Oxidative Stress Injury and Improves Protein Synthesis in Mouse Skeletal Muscle by Regulating the SIRT1/Nrf2/HO-1 Axis and AMPK/TSC2/mTOR/4EBP1 Pathway
- PMID: 40660422
- PMCID: PMC12260125
- DOI: 10.1111/1750-3841.70286
Whey Protein Mitigates Oxidative Stress Injury and Improves Protein Synthesis in Mouse Skeletal Muscle by Regulating the SIRT1/Nrf2/HO-1 Axis and AMPK/TSC2/mTOR/4EBP1 Pathway
Abstract
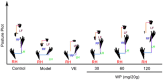
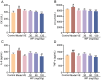
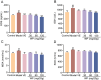
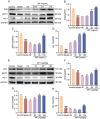
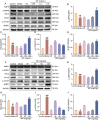
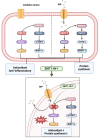
Whey protein (WP) can improve muscle mass and strength. However, its effects and underlying molecular mechanism in promoting recovery from muscle damage caused by excessive physical exercise remains unknown. Therefore, the present study aimed to investigate the therapeutic effect of WP on skeletal muscle injury caused by exogenous oxidants and excessive physical exercise and the potential underlying mechanism. An oxidative stress injury model of mouse skeletal muscle cells was established using hydrogen peroxide (H2O2) and excessive physical exercise. The results revealed that WP effectively improved the migration and differentiation of C2C12 cells exposed to H2O2. Moreover, WP significantly increased the body weight of mice following excessive physical exercise. It also reduced food intake, improved behavioral parameters, enhanced skeletal muscle morphology and function, and promoted protein synthesis, thereby alleviating oxidative stress injury in skeletal muscles. The results further indicated that the mechanism underlying the mitigation of oxidative stress injury in skeletal muscles may involve the silent information regulator sirtuin 1 (SIRT1)/ NF-E2-related factor-2 (Nrf2)/ hemeoxygenase-1 (HO-1) axis. This axis could, in turn, activates the AMP-activated protein kinase (AMPK)/tuberous sclerosis complex subunit 2 (TSC2)/mammalian target of rapamycin (mTOR)/4E-binding protein 1 (4EBP1) pathway, thereby promoting protein synthesis and improving the physiological function of skeletal muscles. This study provides important insights into the role of WP in promoting recovery from muscle damage, offering a basis for future research on WP-based nutritional intervention strategies.
Keywords: AMPK/TSC2/mTOR/4EBP1 pathway; C2C12 cell; Oxidative stress; SIRT1/Nrf2/HO‐1 axis; Skeletal muscle; Whey protein.
© 2025 The Author(s). Journal of Food Science published by Wiley Periodicals LLC on behalf of Institute of Food Technologists.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Buzhong Yiqi decoction improves inflammation and oxidative damage in autoimmune thyroiditis by inhibiting apoptosis via the SIRT1-Mediated Nrf2/NF-κB axis.J Ethnopharmacol. 2025 Jul 24;351:119967. doi: 10.1016/j.jep.2025.119967. Epub 2025 May 11. J Ethnopharmacol. 2025. PMID: 40360040
-
A systematic review of p53 regulation of oxidative stress in skeletal muscle.Redox Rep. 2018 Dec;23(1):100-117. doi: 10.1080/13510002.2017.1416773. Epub 2018 Jan 3. Redox Rep. 2018. PMID: 29298131 Free PMC article.
-
miR-210 Regulates Autophagy Through the AMPK/mTOR Signaling Pathway, Reduces Neuronal Cell Death and Inflammatory Responses, and Enhances Functional Recovery Following Cerebral Hemorrhage in Mice.Neurochem Res. 2025 Jun 5;50(3):180. doi: 10.1007/s11064-025-04434-7. Neurochem Res. 2025. PMID: 40471451 Free PMC article.
-
[Eccentric treadmill exercise promotes adaptive hypertrophy of gastrocnemius in rats].Sheng Li Xue Bao. 2025 Jun 25;77(3):449-464. doi: 10.13294/j.aps.2025.0051. Sheng Li Xue Bao. 2025. PMID: 40566712 Chinese.
-
The Effect of Whey Protein Supplementation on the Temporal Recovery of Muscle Function Following Resistance Training: A Systematic Review and Meta-Analysis.Nutrients. 2018 Feb 16;10(2):221. doi: 10.3390/nu10020221. Nutrients. 2018. PMID: 29462923 Free PMC article.
References
-
- Ataei, L. , Giannaki C. D., Petrou C., and Aphamis G.. 2023. “Effect of Tribulus Terrestris L. Supplementation on Exercise‐Induced Oxidative Stress and Delayed Onset Muscle Soreness Markers: A Pilot Study.” Journal of Dietary Supplements 20, no. 6: 811–831. 10.1080/19390211.2022.2120147. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

