Unveiling Crocosphaera Responses to Phosphorus Depletion: Insights From Genome Analysis and Functional Characterization
- PMID: 40660640
- PMCID: PMC12260270
- DOI: 10.1111/1462-2920.70153
Unveiling Crocosphaera Responses to Phosphorus Depletion: Insights From Genome Analysis and Functional Characterization
Abstract
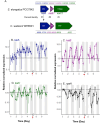
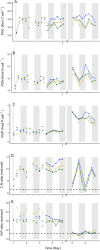
Unicellular, nitrogen-fixing cyanobacteria (UCYN) thrive and support primary production in oligotrophic oceans, playing a significant role in the marine nitrogen cycle. Crocosphaera sp., a model organism for studying marine nitrogen fixation, is adapted to low phosphate (Pi) concentrations. Yet, how Crocosphaera copes with Pi depletion is rather poorly understood. We present a genomics analysis of Pi stress-responsive genes in this genus, encompassing six C. watsonii and two strains isolated in coastal environments, C. subtropica and C. chwakensis. We identified genes involved in Pi signalling and uptake, and dissolved organic phosphorus (DOP) hydrolysis. Results showed different genetic potentials to cope with Pi scarcity between the Crocosphaera strains. Physiological monitoring of cultures of C. watsonii WH8501 exposed to Pi depletion highlighted a capacity to survive for at least nine days, albeit with a skewed C:N:P stoichiometry. Upon addition of DOP, cultures efficiently recovered to a growth rate and cell composition equivalent to those observed under favourable conditions. The concomitant transcription analysis revealed diel expression patterns of Pi-related genes and endogenous clock genes, suggesting a possible circadian regulation. Our data deepen our understanding of the growth strategies Crocosphaera employs in Pi-limited environments, offering broader insights into microbial resilience in marine ecosystems.
Keywords: Crocosphaera; cyanobacteria; diel cycle; gene expression; genomics; phosphorus depletion.
© 2025 The Author(s). Environmental Microbiology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Bell, D. W. , Pellechia P. J., Ingall E. D., and Benitez‐Nelson C. R.. 2020. “Resolving Marine Dissolved Organic Phosphorus (DOP) Composition in a Coastal Estuary.” Limnology and Oceanography 65: 2787–2799.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

