Heavy Menstrual Bleeding and Hormonal Therapy in Women with Type 1 von Willebrand Disease Enrolled on the Zimmerman Program
- PMID: 40660798
- PMCID: PMC12264403
- DOI: 10.1177/10760296251359294
Heavy Menstrual Bleeding and Hormonal Therapy in Women with Type 1 von Willebrand Disease Enrolled on the Zimmerman Program
Abstract
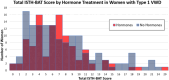
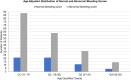
IntroductionWomen with von Willebrand Disease (VWD) frequently experience heavy menstrual bleeding (HMB), impacting their health and quality of life. Hormonal contraceptives are a standard treatment.AimWe hypothesized that women with type 1 VWD using hormonal therapy would have lower bleeding scores and potentially higher VWF levels than non-users. To test this, we compared demographics, VWF levels, and International Society on Thrombosis and Haemostasis Bleeding Assessment Tool (ISTH-BAT) scores between hormone users and non-users in the Zimmerman Program cohort.MethodsData from 269 women with type 1 VWD enrolled in the Zimmerman Program were analyzed. Of these, 103 were hormone users, including oral contraceptives (71), medroxyprogesterone acetate (11), levonorgestrel IUD (11), contraceptive implant (3), and the etonogestrel/ethinyl estradiol vaginal ring (1) and (6) subjects were undergoing hormone replacement therapy. Participants who were postmenopausal were excluded from the study results.ResultsHMB was reported by 252 out of 269 participants (94%), with 118 (47%) reporting severe symptoms. While hormone users were younger than non-users, 22.3 versus 23.2 years, (p = .003), VWF levels were similar between groups, (0.36 vs 0.38IU/mL). Age-adjusted BAT scores revealed that in all age groups, more than 70% of patients had abnormal bleeding scores.ConclusionHormonal therapy users had lower total BAT scores compared to non-users, though differences were not statistically significant. These findings suggest a potential but limited effect of hormone use on bleeding phenotype in women with type 1 VWD.
Keywords: blood coagulation disorder inherited; contraceptive agents; hemorrhage; hormonal; menorrhagia; type 1; von willebrand disease.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Nicoletta Machin has received research funding from Takeda USA and serves on advisory boards for Sanofi and Star Therapeutics. Margaret Ragni has received research funding to the University from Sanofi, BioMarin, SPARK, and Takeda USA, and serves on advisory boards for Sanofi, BeBio, HEMAB, SPARK, and Takeda USA. Veronica Flood has received research funding from Octapharma. Anthony Navarrete, Pamela Christopherson, and Robert Montgomery have no conflicts of interest to declare related to this research.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

