Molecular imaging using (nano)probes: cutting-edge developments and clinical challenges in diagnostics
- PMID: 40661211
- PMCID: PMC12257482
- DOI: 10.1039/d5ra03927d
Molecular imaging using (nano)probes: cutting-edge developments and clinical challenges in diagnostics
Abstract
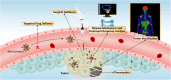
Molecular imaging has emerged as a transformative approach in the field of medical diagnostics, enabling the visualization of biological processes at the molecular and cellular levels. Additionally, the integration of molecular imaging with other imaging modalities such as positron emission tomography (PET), magnetic resonance imaging (MRI), computed tomography (CT), photoacoustic imaging (PAI), and fluorescence imaging (FI) has further broadened the scope of diagnostics. Despite significant advances in probe design, including multifunctional and targeted nanomaterials, their clinical translation remains limited by critical challenges. Key obstacles include nanoprobe stability in physiological environments, nonspecific accumulation in the reticuloendothelial system, potential toxicity, and difficulties in achieving optimal biocompatibility and controlled biodistribution. Moreover, the complexity of nanoprobe synthesis and batch-to-batch variability hinder scalable manufacturing and regulatory approval. The primary goal of this review is to critically analyze the current challenges hindering the clinical translation of molecular imaging nanoprobes in biomedicine. While existing literature extensively covers imaging techniques, this review uniquely emphasizes the persistent obstacles-such as nanoprobe stability, biocompatibility, off-target effects, and limited sensitivity-that impede their effective application. Unlike previous reviews, which tend to focus broadly on advancements, we offer a nuanced perspective by identifying specific barriers and proposing promising strategies to overcome them. We explore how surface modification, novel targeting ligands, and smart responsive systems can enhance nanoprobe performance. Furthermore, the review discusses how addressing these challenges is crucial for accelerating the development of multifunctional nanoprobes capable of simultaneous diagnosis and therapy, ultimately advancing personalized medicine. By highlighting these hurdles and potential solutions, this review aims to provide a comprehensive roadmap for researchers striving to optimize molecular imaging nanoprobes, thereby bridging the gap between laboratory innovation and clinical reality.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Author(s) declare no conflict of interest.
Figures








Similar articles
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
A Spectrum of Understanding: A Qualitative Exploration of Autistic Adults' Understandings and Perceptions of Friendship(s).Autism Adulthood. 2024 Dec 2;6(4):438-450. doi: 10.1089/aut.2023.0051. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018059
-
Neonatal Nurses' Understanding of the Factors That Enhance and Hinder Early Communication Between Preterm Infants and Their Parents: A Narrative Inquiry Study.Int J Lang Commun Disord. 2025 Jul-Aug;60(4):e70093. doi: 10.1111/1460-6984.70093. Int J Lang Commun Disord. 2025. PMID: 40653954 Free PMC article.
References
-
- MacRitchie N. Frleta-Gilchrist M. Sugiyama A. Lawton T. McInnes I. B. Maffia P. Pharmacol. Ther. 2020;211:107550. - PubMed
-
- Jamalipour Soufi G. Hekmatnia A. Iravani S. Varma R. S. ACS Appl. Nano Mater. 2022;5:10151–10166.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

