This is a preprint.
COVID-19 Vaccination Induces Cross-Reactive Dengue Antibodies with Altered Isotype Profiles and In Vitro ADE
- PMID: 40661288
- PMCID: PMC12258759
- DOI: 10.1101/2025.05.23.25328123
COVID-19 Vaccination Induces Cross-Reactive Dengue Antibodies with Altered Isotype Profiles and In Vitro ADE
Abstract
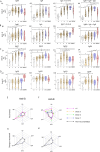
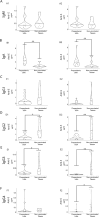
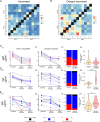
Dengue virus is a mosquito transmitted flavivirus that cause inapparent or mild disease but can also cause severe hemorrhagic fever in humans. Cross-reactive IgG antibodies can cause under specific conditions antibody dependent enhancement (ADE) of the infection via Fc-receptor interaction. Antibodies from SARS-CoV-2 (anti-S) causing COVID-19 disease can also cross-react against the dengue envelope (anti-E). We therefore investigated the antibody profile of these cross-reactive antibodies and their ability to induce ADE. We found that the cross-reactive anti-E in COVID-19 vaccinated are dominated by IgM/A while, anti-S from vaccinated or anti-E from dengue infected patients have an IgG1 dominated antibody profile. The low-level anti-E IgG from COVID-19 vaccinated are dominated by low Fc-affinity IgG2/4 subclass. These antibodies were able to induce in vitro ADE stronger than from Dengue infected for the 1st,2nd dose of the vaccine or later omicron variant booster. This could partially be explained that ADE was inhibited by the complement system in dengue infected but not in COVID-19 vaccinated.
Keywords: Cross-reactive; Dengue; antibody dependent enhancement; isotype.
Conflict of interest statement
Conflict of interests Etsuro Ito is member of the R&D Department, BioPhenoMA Inc., Tokyo, Japan.
Figures

Similar articles
-
COVID-19 Vaccines.2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. 2025 Jul 15. Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006–. PMID: 33355732 Free Books & Documents. Review.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Sexual Harassment and Prevention Training.2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Mar 29. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 36508513 Free Books & Documents.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
-
- Sarker S, Dutta C, Mallick A, et al. Dengue virus (DV) non-cross-reactive Omicron wave COVID-19 serums enhanced DV3 infectivity in vitro. J Med Microbiol. 2025;73(3). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
