This is a preprint.
Hibernation improves neural performance during energy stress in regions across the central nervous system in the American bullfrog
- PMID: 40661346
- PMCID: PMC12259159
- DOI: 10.1101/2025.06.13.659541
Hibernation improves neural performance during energy stress in regions across the central nervous system in the American bullfrog
Abstract
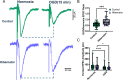
Neuronal signaling requires high rates of ATP production via the oxidative metabolism of glucose. The American bullfrog is intriguing, as this species has typical brain energy requirements for an average vertebrate but modifies synaptic physiology and metabolism after hibernation to maintain function during hypoxia and ischemia. Given the importance of the respiratory system in restoring metabolic homeostasis during emergence from underwater hibernation, work to date has addressed this response in the brainstem respiratory network. Thus, metabolic plasticity has been interpreted as an adaptation used to restart respiratory motor behavior under hypoxic conditions during the transition from skin breathing to air breathing. It remains unclear whether these improvements are specific to the brainstem regions critical for breathing versus a global response within the central nervous system (CNS). To address this question, we recorded neural activity from the spinal cord, forebrain, and brainstem respiratory network in vitro. As expected, hypoxia disrupted the function of each network in control animals. After hibernation, each network improved its activity in hypoxia compared to controls. These results suggest that plasticity that improves neural function during energy stress following hibernation reflects a global response that may impact many behaviors controlled by the CNS and is not limited to regions involved in metabolic homeostasis.
Conflict of interest statement
Conflict of Interests: The authors declare no conflicts of interest.
Figures



Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Ventilator Management(Archived).2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 28846232 Free Books & Documents.
-
Systemic Inflammatory Response Syndrome.2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jun 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31613449 Free Books & Documents.
-
Physical interventions to interrupt or reduce the spread of respiratory viruses.Cochrane Database Syst Rev. 2023 Jan 30;1(1):CD006207. doi: 10.1002/14651858.CD006207.pub6. Cochrane Database Syst Rev. 2023. PMID: 36715243 Free PMC article.
References
-
- Adams S., Zubov T., Bueschke N., & Santin J. M. (2021). Neuromodulation or energy failure? Metabolic limitations silence network output in the hypoxic amphibian brainstem. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 320(2), R105–R116. 10.1152/ajpregu.00209.2020 - DOI - PMC - PubMed
-
- Baghdadwala M. I. (2016). Diving into the mammalian swamp of respiratory rhythm generation with the bullfrog. Respiratory Physiology. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
