This is a preprint.
The cholinergic drug galantamine ameliorates acute and subacute peripheral and brain manifestations of acute respiratory distress syndrome in mice
- PMID: 40661460
- PMCID: PMC12258725
- DOI: 10.1101/2025.05.17.654675
The cholinergic drug galantamine ameliorates acute and subacute peripheral and brain manifestations of acute respiratory distress syndrome in mice
Update in
-
The cholinergic drug galantamine ameliorates acute and subacute peripheral and brain manifestations of acute respiratory distress syndrome in mice.Sci Rep. 2025 Sep 26;15(1):33217. doi: 10.1038/s41598-025-18542-5. Sci Rep. 2025. PMID: 41006538 Free PMC article.
Abstract
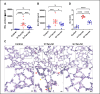
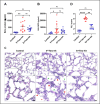
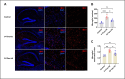
Acute respiratory distress syndrome (ARDS) is a life-threatening form of acute lung injury (ALI), which is a common cause of respiratory failure and high mortality in critically ill patients. Long-term mortality and brain dysfunction have been documented in ARDS patients after hospital discharge. Inflammation plays a key role in ALI/ARDS pathogenesis. Neural cholinergic signaling regulates cytokine responses and inflammation. Here, we studied the effects of galantamine, an approved cholinergic drug (for Alzheimer's disease) on ALI/ARDS severity and inflammation in mice, using a clinically relevant mouse model induced by intratracheal administration of hydrochloric acid and lipopolysaccharide. Mice were treated 30 mins prior to each insult with vehicle or galantamine (4 mg/kg, i.p.). Galantamine treatment significantly decreased bronchoalveolar lavage (BAL) and serum TNF, IL-1β, and IL-6 levels, as well as BAL total protein and myeloperoxidase and lung histopathology in ALI/ARDS mice. In addition, galantamine improved the functional state of mice with ALI/ARDS during a 10-day monitoring and attenuated lung injury and indices of brain inflammation at 10 days. These findings support further studies utilizing this approved cholinergic drug in therapeutic strategies for ARDS and its subacute sequelae.
Keywords: acute lung injury; acute respiratory distress syndrome; brain; cholinergic signaling; galantamine; inflammation.
Conflict of interest statement
Declaration of interests VAP and KJT have co-authored patents broadly related to the content of this paper. They have assigned their rights to the Feinstein Institutes for Medical Research. AF, SPP, SC, AT, FMC, CNM, MB, EHC, and SSC declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
