This is a preprint.
Swine reporter model for preclinical evaluation and characterization of gene delivery vectors
- PMID: 40661506
- PMCID: PMC12259160
- DOI: 10.1101/2025.06.13.659546
Swine reporter model for preclinical evaluation and characterization of gene delivery vectors
Abstract
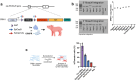
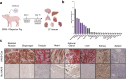
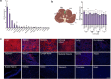
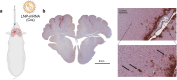
Delivery of gene therapy vectors targeted to any somatic cell remains a key barrier for the development of genetic medicines. While rodent models provide insights into vector biodistribution and cellular tropism, their anatomical and physiological differences from humans limit their translational potential and studies in large animal models are often required. In this study, we developed a swine reporter model (SRM-1) to evaluate both viral and nonviral vector delivery in a large animal system. The SRM-1 model harbors a Cre- and CRISPR-activated tdTomato reporter at the Rosa26 locus that allows for tracing of cell-specific delivery and expression of gene therapy vectors in vivo. To evaluate this model, we administered adeno-associated virus serotype 9 (AAV9) and lipid nanoparticles (LNPs) carrying mRNA systemically and found successful in vivo reporter activation across a variety of tissues. Intracerebroventricular (ICV) administration of LNP-Cre mRNA was also performed and demonstrated localized activation in cortical brain cells. In addition to biodistribution studies, this model has utility for testing safety and clinically relevant administration methods, surgical and non-surgical, of delivery vectors. Our findings support the SRM-1 model as a valuable tool for advancing gene therapies from preclinical testing to clinical application.
Conflict of interest statement
JMC, DMK, HD, DAW, ROA, and DMC are current employees of Recombinetics, which seeks to commercialize the SRM-1 model. N.M. and H.H. are founders of Opus Biosciences. J.K.W. is a co-founder of Nucyrna Therapeutics and an advisory board member of EnPlusOne, PepGen, and Sixfold.
Figures

References
-
- Staahl B.T., Benekareddy M., Coulon-Bainier C., Banfal A.A., Floor S.N., Sabo J.K., Urnes C., Munares G.A., Ghosh A., and Doudna J.A. (2017). Efficient genome editing in the mouse brain by local delivery of engineered Cas9 ribonucleoprotein complexes. Nat Biotechnol 35, 431–434. 10.1038/nbt.3806. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
