Applying a logistic regression-clustering joint model to analyze the causes of prolonged pre-analytic turnaround time for urine culture testing in hospital wards
- PMID: 40661657
- PMCID: PMC12256443
- DOI: 10.3389/fdgth.2025.1603314
Applying a logistic regression-clustering joint model to analyze the causes of prolonged pre-analytic turnaround time for urine culture testing in hospital wards
Abstract
Introduction: In this study, we developed and validated a logistic regression-clustering joint model to: (1) quantify multistage workflow bottlenecks (collection/transport/reception) in urine culture pre-TAT prolongation (>115 min); and (2) assess the efficacy of targeted interventions derived from model-derived insights.
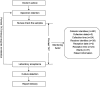
Methods: Using complete workflow data obtained from 1,343 urine culture specimens (January 2024-March 2024) collected at a tertiary hospital, we integrated binary logistic regression analysis with K-means clustering to quantify delay patterns. The analyzed variables included collection time, ward type, personnel roles, and patient demographics. Post-intervention data (May 2024-July 2024, *n* = 1,456) was also analyzed to assess the impact.
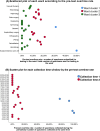
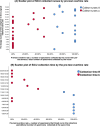
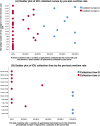
Results: Analysis of the critical risk factors revealed that specimens collected between 04:00-05:59/10:00-11:59 had 142.92-fold higher delay odds (95% CI: 58.81-347.37). Those collected on SICU/ICU wards showed 9.98-fold higher risk (95% CI: 5.05-19.72) than general wards. Regarding intervention efficacy, pre-TAT overtime rates decreased by 58.6% (13.48% → 7.55%, P < 0.01). Contamination rate decreased by 59.8% (5.67% → 2.28%, P < 0.01). The median pre-TAT decreased by 15.9% (44 → 37 min, P < 0.01).
Discussion: The joint model effectively identified workflow bottlenecks. Targeted interventions (dynamic transport scheduling, standardized training, and IoT alert systems) significantly optimized pre-TAT and specimen quality, providing a framework for improving clinical laboratory processes.
Keywords: logistic regression model; medical quality control; pre-analytical turnaround time (pre-TAT); process optimization; urine microbial culture.
© 2025 Lv, Ye, Li and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Pankawase JM, Shinde V. Effect of pre-analytical errors on turnaround time of central clinical laboratory: a study protocol. ECS Trans. (2022) 107:17457. 10.1149/10701.17457ecst - DOI
-
- LaRocco MT, Franek J, Leibach EK, Weissfeld AS, Kraft CS, Sautter RL, et al. Effectiveness of preanalytic practices on contamination and diagnostic accuracy of urine cultures: a laboratory medicine best practices systematic review and meta-analysis. Clin Microbiol Rev. (2016) 29:105–47. 10.1128/CMR.00030-15 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

