Effects of Exosomes From Hypoxia-Induced Adipose-Derived Stem Cells on Ameliorating Photoaging
- PMID: 40661769
- PMCID: PMC12257001
- DOI: 10.2147/CCID.S523936
Effects of Exosomes From Hypoxia-Induced Adipose-Derived Stem Cells on Ameliorating Photoaging
Abstract
Introduction: Photoaging, a significant concern in cosmetic dermatology, involves complex skin damage that necessitates effective treatments. Exosomes derived from adipose-derived stem cells (ADSCs), particularly those generated under hypoxic conditions (hypADSC-Exo), have emerged as a promising cell-free therapeutic approach. This study investigates the effects of hypADSC-Exo on reducing human dermal fibroblast (HDF) senescence and mitigating signs of photoaging through topical application in a mouse model.
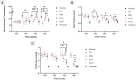
Methods: Exosomes were isolated from hypoxia-induced human ADSCs via ultracentrifugation and identified using flow cytometry (CD9, CD63, CD81). Transmission electron microscopy (TEM) confirmed the vesicle morphology, while the Bradford assay and nanoparticle tracking analysis (NTA) assessed the protein content and size. In vitro, UV-induced senescent HDFs were treated with hypADSC-Exo. Cell morphology, senescence (SA-β-gal assay), proliferation (Alamar Blue), and gene expression (p16, p21 via qPCR) were evaluated. In vivo, photoaged mice received hypADSC-Exo treatments (50 or 100 μg/mL) twice weekly for six weeks. Skin parameters (wrinkles, thickness, hydration, elasticity) were evaluated biweekly. Skin biopsies were used to assess epidermal and dermal thickness, collagen density, and gene expression of collagen types 1, 3 and MMP-1, 2, and 3.
Results: hypADSC-Exo exhibited a cup-shaped morphology under TEM and expressed exosomal markers CD9, CD63, and CD81. In vitro, hypADSC-Exo improved HDF morphology, reduced SA-β-gal activity, enhanced proliferation, and downregulated p16 and p21. In vivo, it reduced skin wrinkles and thickness. Treated mice exhibited improvement in hydration, elasticity, decreased epidermal and dermal thickness, and increased collagen density. Collagen types 1 and 3 increased slightly, while the levels of MMP-1, 2, and 3 decreased in the exosome group.
Conclusion: Our findings demonstrate that hypADSC-Exo reduces senescence in UV-induced aged HDF and improves photoaging in mice. These effects likely result from decreased MMP-1, 2, 3 expression and increased collagen deposition, making hypADSC-Exo a promising therapy for photoaging.
Keywords: adipose-derived stem cell; hypoxic exosome; photoaging; senescent dermal fibroblast.
© 2025 Huynh et al.
Conflict of interest statement
The authors declare no competing interests, financial or otherwise, associated with this publication.
Figures






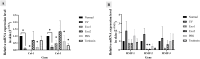
References
-
- Katta R, Kramer MJ. Skin and Diet: an Update on the Role of Dietary Change as a Treatment Strategy for Skin Disease. Skin Therapy Lett. 2018;23(1):1–5. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

