Proteostasis Disruption by Proteasome Inhibitor MG132 and Propolin G Induces ER Stress- and Autophagy-Mediated Apoptosis in Breast Cancer
- PMID: 40661805
- PMCID: PMC12256276
- DOI: 10.1002/fsn3.70632
Proteostasis Disruption by Proteasome Inhibitor MG132 and Propolin G Induces ER Stress- and Autophagy-Mediated Apoptosis in Breast Cancer
Abstract
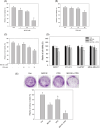
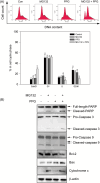
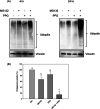
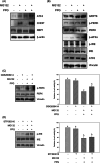
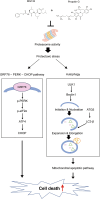
The maintenance of protein homeostasis, commonly referred to as proteostasis, is critical for the proper functioning of cells. Disruptions in protein degradation pathways can result in proteotoxic stress, which may ultimately lead to cellular apoptosis. Targeting the dysregulation of proteostasis has emerged as a promising approach in cancer therapy. Propolin G, a c-prenylflavanone derived from Taiwanese propolis, has demonstrated anticancer properties; however, its underlying mechanisms remain largely unexplored. In this study, we evaluated the combined effect of propolin G and the proteasome inhibitor MG132 on breast cancer cells. While individual treatments with MG132 (1 μM) or propolin G (10 μM) exhibited minimal effects on cell viability, their combination resulted in a synergistic suppression of proliferation and induction of apoptosis, as indicated by a combination index (CI) of 0.63. This combined treatment significantly reduced proteasome activity, leading to the accumulation of polyubiquitinated proteins. Mechanistically, apoptosis was mediated through the activation of the PERK/ATF4/CHOP signaling pathway and autophagy, as evidenced by increased expression levels of ULK1, Beclin1, ATG5, and LC3-II. These findings highlight the potential of targeting proteostasis disruption as an effective anticancer strategy in breast cancer. The combination of propolin G and MG132 may leverage cancer-specific vulnerabilities and possess translational potential for anticancer therapy.
Keywords: autophagy; propolin G; proteasome inhibitor; protein homeostasis.
© 2025 The Author(s). Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

