Retrospective Cohort Analysis of Clinical, Molecular, and Histopathologic Characteristics of 275 Patients With Nemaline Myopathy
- PMID: 40661861
- PMCID: PMC12258819
- DOI: 10.1212/NXG.0000000000200277
Retrospective Cohort Analysis of Clinical, Molecular, and Histopathologic Characteristics of 275 Patients With Nemaline Myopathy
Abstract
Background and objectives: Nemaline myopathy (NM) is a congenital myopathy with a wide severity spectrum, from severe, generalized muscle weakness and respiratory failure in the neonatal period to mild, distal weakness in young adulthood. Eleven genes have been definitively established to cause the condition. Although some recurrent variants have been identified, the overall correlation of genotype with clinical severity in NM remains poor. This poses challenges when counseling families about prognosis after a diagnosis of NM is made. Better clinical and molecular predictors of outcome are needed for clinical trial readiness.
Methods: A retrospective cohort analysis of 275 patients with a histopathologic diagnosis of NM and/or pathogenic variants in NM-associated genes was performed to identify relationships between early clinical findings and long-term outcomes, including need for respiratory and feeding support.
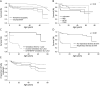
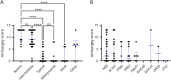
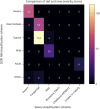
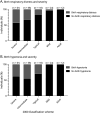
Results: Early clinical predictors of long-term outcomes were identified: patients with hypotonia at birth had increased odds of requiring gastrostomy tubes, and patients with respiratory distress at birth had increased odds of requiring both gastrostomy tubes and invasive ventilation. Individuals with ACTA1-NM were more likely to require feeding tubes and invasive ventilation in the first year of life compared with those with NEB-related NM, but the odds of requiring invasive ventilation were similar after the first year of age. Additional clinical information is presented by genotype and severity class.
Discussion: Neonatal findings of individuals with NM are correlated with long-term clinical outcomes, and there are some relationships between genotype and disease severity. Prospective longitudinal studies are needed to confirm these findings and evaluate for additional early clinical predictors of prognosis.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Conflict of interest statement
B.T. Darras has served as an ad hoc scientific advisory board member for Audentes Therapeutics, AveXis/Novartis Gene Therapies, Biogen, Pfizer, Sarepta, Vertex and Roche/Genentech; was a Steering Committee Chair for Roche FIREFISH and MANATEE studies and data safety monitoring board member for Amicus Inc. and Lexeo Therapeutics; he has no financial interests in these companies. He has received research support from the NIH/NINDS, the Slaney Family Fund for SMA, the Spinal Muscular Atrophy Foundation, CureSMA, and Working on Walking Fund and has received grants from Ionis Pharmaceuticals, Inc., for the ENDEAR, CHERISH, CS2/CS12 studies; from Biogen for CS11; and from AveXis, Sarepta Pharmaceuticals, Novartis (AveXis), PTC Therapeutics, Roche, Scholar Rock, and Fibrogen and has also received royalties for books and online publications from Elsevier and UpToDate, Inc. S. Iannaccone recieves research funding from NIH and DoD W81X WH 2010293, Cure SMA and industry (Astellas, RegenxBio, Capricor, Novartis, Biogen, Sarepta, PTC Therapeutics and Scholar Rock). She has served on medical advisory boards for Novartics, Biogen and Sarepta and as consultant for Genetech. L.H. Hayes reports research funding from Vertex Pharmaceuticals, Astellas Pharma, Novartis, and Biohaven Pharmaceuticals, and recieves consulting fees from Biohaven Pharmaceuticals. A.H. Beggs reports grants or contracts for studies of skeletal muscle and diagnosis and treatment of congenital myopathies from NIH, MDA (USA), A Foundation Building Strength, and the Chan Zuckerberg Initiative, and from AFM Telethon, Alexion Pharmaceuticals Inc., Avidity, Dynacure SAS, Kate Therapeutics, and Pfizer Inc. He has received consulting fees from Astellas Pharma, F. Hoffmann-La Roche, GLG, Guidepoint Global, and Kate Therapeutics; has received support for attending meetings from Kate Therapeutics and Muscular Dystrophy Association; holds equity in Kate Therapeutics and Kinea Bio; and is an inventor on a US patent describing a method for gene therapy of X-linked myotubular myopathy. The remaining authors report no disclosures relevant to the manuscript.Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/NG.
Figures


References
-
- Wallgren-Pettersson C, Beggs AH, Laing NG. 51st ENMC international workshop: nemaline myopathy. 13-15 June 1997, Naarden, The Netherlands. Neuromuscul Disord. 1998;8(1):53-56. - PubMed
LinkOut - more resources
Full Text Sources
