CD8+ T cells promote tubule-interstitial damage in malaria-induced acute kidney injury
- PMID: 40661967
- PMCID: PMC12257197
- DOI: 10.3389/fcimb.2025.1561806
CD8+ T cells promote tubule-interstitial damage in malaria-induced acute kidney injury
Abstract
Introduction: Malaria acute kidney injury (MAKI) is associated with severe malaria and correlates with poor prognosis and death of patients infected with Plasmodium falciparum. The pathogenesis of MAKI is not completely understood but some hypotheses are well recognized. Host-parasite interactions lead to mechanical obstruction, disorders in the renal microcirculation, and immune-mediated glomerular injury. We investigated the influence of CD8⁺ T cells in the pathogenesis of malaria-induced renal disease.
Methods: To assess the role of T lymphocytes in MAKI pathogenesis, we used adoptive transfer; antibody-driven CD8+ T cells depletion and treatment with FYT720.
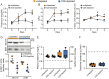
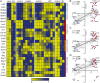
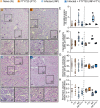
Results: The transference of total T cells isolated from malaria-infected donor mice into naive recipient animals reproduced kidney tubule-interstitial damage without affecting glomerular function. It was associated with increased accumulation of CD8+ T cells in the kidneys of recipient mice. The selective depletion of CD8+ T cells in infected animals resulted in protection from tubular injury, although glomerular alterations still occurred. Finally, we evaluated FTY720, an immunomodulatory drug that sequesters T cells in lymphoid organs and limits their migration, as a potential therapeutic strategy. Treatment with FTY720 prevented the development of proteinuria and the increase in the urine to creatinine ratio. Moreover, FTY720 reduced urinary γ-glutamyl transferase (γ-GT) levels, a marker of tubular injury, but did not alter plasma urea and creatinine, two markers of glomerular function.
Discussion: Our results add new knowledge demonstrating that CD8+ T cells have a specific role in tubule-interstitial injury pathology during MAKI.
Keywords: CD8+ T cells; Plasmodium; acute kidney injury; host defense; host-pathogen interaction; malaria; tubule-interstitial injury.
Copyright © 2025 Teixeira, Alves, Pinheiro, Silva, Silva-Aguiar, Peruchetti, Pádua, Souza, Caruso-Neves and Pinheiro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Batte A., Berrens Z., Murphy K., Mufumba I., Sarangam M. L., Hawkes M. T., et al. (2021). Malaria-associated acute kidney injury in african children: prevalence, pathophysiology, impact, and management challenges. Int. J. Nephrol. Renovasc Dis. Volume 14, 235–253. doi: 10.2147/IJNRD.S239157 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

