Short-Term Effects of Tirzepatide in Obese Adults: A Real-World Prospective Study
- PMID: 40662047
- PMCID: PMC12256807
- DOI: 10.7759/cureus.85970
Short-Term Effects of Tirzepatide in Obese Adults: A Real-World Prospective Study
Abstract
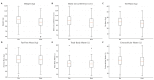
Background Tirzepatide is a novel dual agonist of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors, shown to induce substantial weight loss in clinical trials. However, real-world data on the short-term effects of the drug in obese adults is unknown. This prospective observational study aimed to evaluate the short-term effects of tirzepatide on body composition-including fat mass, fat-free mass, total body water, and extracellular water-as well as waist circumference and body weight in adults with overweight or obesity under routine clinical care. Methods Adults with a body mass index (BMI) ≥27 kg/m² who were newly prescribed tirzepatide received once-weekly subcutaneous injections, alongside standardized lifestyle counseling. Body composition parameters, including fat mass (FM), fat-free mass (FFM), total body water (TBW), and extracellular water (ECW), were assessed using bioelectrical impedance analysis (BIA) under standardized conditions at baseline and after a median of 30 days. The study was conducted between December 1, 2024, and May 1, 2025. Changes from baseline were analyzed using the Wilcoxon signed-rank test; subgroup analyses were performed by sex and age. Spearman's rank correlation coefficients were used to examine associations between age and treatment response. Results A total of 115 participants (67.5% female; median age 48 years) were included. Median body weight decreased by -4.0 kg, waist circumference by -5.0 cm, and fat mass by -3.6 kg. Changes in fat-free mass and total body water were minimal, suggesting preferential loss of adipose tissue. Although females demonstrated a slightly greater median reduction in body weight [median: -4.0 kg; interquartile range (IQR): -7.0 to -2.1 kg] compared to males (median: -3.0 kg; IQR: -6.0 to 0.0 kg), the difference was not statistically significant (p = 0.112). Similarly, no statistically significant differences were observed between sexes for the change in total body water [difference in TBW (DTBW): median for females: 0.1 L vs. -0.4 L for males, p = 0.733], extracellular water [difference in ECW (DECW): -0.6 L vs. 0.5 L, p = 0.240], or FFM [difference in FFM (DFFM): -0.7 kg vs. -0.6 kg, p = 0.276]. Age was not correlated with body composition changes. Conclusion Tirzepatide was associated with rapid and clinically meaningful reductions in body weight, central adiposity, and fat mass within 30 days, with preservation of lean mass, supporting its utility in early obesity management.
Keywords: body composition; fat mass; obesity; tirzepatide; weight loss.
Copyright © 2025, Adamidis et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. IRB of Athens Medical Group issued approval 106/28-11-2024. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
LinkOut - more resources
Full Text Sources
