Chemotherapy and radiotherapy use in patients with lung cancer in Australia, Canada, the UK and Norway 2012-2017: an ICBP population-based study
- PMID: 40662158
- PMCID: PMC12258358
- DOI: 10.1136/bmjonc-2025-000800
Chemotherapy and radiotherapy use in patients with lung cancer in Australia, Canada, the UK and Norway 2012-2017: an ICBP population-based study
Abstract
Background: International variation in lung cancer survival may be partly explained by variation in stage-specific treatment use, but relevant comparative evidence is sparse. As part of the International Cancer Benchmarking Partnership, we examined use of chemotherapy and radiotherapy in population-based cancer registry data.
Methods: Linked population-based data sources were used to describe use and time to first treatment for either chemotherapy or radiotherapy in patients with lung cancer diagnosed in study periods during 2012-2017 in 16 jurisdictions of Australia, Canada, the UK and Norway.
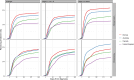
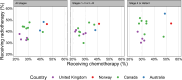
Results: There was large variation in the proportions of patients with lung cancer receiving chemotherapy (ranging from 23% in Northern Ireland to 45% in Norway) and radiotherapy (ranging from 32% in England to 48% in New South Wales and 50% in Newfoundland and Labrador). Across jurisdictions, chemotherapy use decreased steeply with increasing age, regardless of stage at diagnosis. For radiotherapy use, in stage 1-3 cancer three patterns were observed: (a) steep decrease with increasing age (UK jurisdictions, Saskatchewan-Manitoba); (b) a relatively flat pattern (Norway, Alberta, British Columbia, Atlantic Canada, New South Wales) and (c) increasing use with increasing age (Ontario).Time to radiotherapy initiation was longer in the UK jurisdictions than elsewhere; time to chemotherapy was longer in the UK and Canadian jurisdictions except Ontario.
Discussion: Use of chemotherapy and radiotherapy in patients with lung cancer varied substantially between jurisdictions during the mid-2010s within age-stage strata. Reasons for these variations are unclear. Differences in non-surgical treatment use are plausibly associated with international variation in lung cancer survival.
Keywords: chemotherapy; epidemiology; lung cancer (non-small cell); lung cancer (small-cell); radiotherapy.
Copyright © Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
MEB reports personal fees from GRAIL Bio UK, for Independent Data Monitoring Committee (IDMC) membership unrelated to this study. OB and GM report salary compensation for analysis of trial data in preparation for review by the Data Safety Monitoring Board for the POWERRANGER trial (NCT01404156), unrelated to this project. DWH reports grant support by Moondance Cancer Initiative (to institution) in relation to exploring bowel cancer audit data. YN reports grant support to The Cancer Registry of Norway by the Norwegian Cancer Society on standardised cancer pathways (no direct payment). RRW reports grant funding by the BC Cancer Foundation for examining cancer outcomes among Indigenous populations in BC, and funding by the Canadian Partnership Against Cancer for data development projects. GL declares research grant funding by the study sponsors to his employer (University College London).
Figures





Comment in
-
Inequities in lung cancer treatment: lessons from international variation in chemotherapy and radiotherapy use.BMJ Oncol. 2025 Aug 28;4(1):e000877. doi: 10.1136/bmjonc-2025-000877. eCollection 2025. BMJ Oncol. 2025. PMID: 40909187 Free PMC article. No abstract available.
References
-
- Araghi M, Fidler-Benaoudia M, Arnold M, et al. ICBP SURVMARK-2 Local Leads, ICBP SURVMARK-2 Academic Reference Group. International differences in lung cancer survival by sex, histological type and stage at diagnosis: an ICBP SURVMARK-2 Study. Thorax. 2022;77:378–90. doi: 10.1136/thoraxjnl-2020-216555. - DOI - PubMed
-
- McPhail S, Barclay ME, Johnson SA, et al. Use of chemotherapy in patients with oesophageal, stomach, colon, rectal, liver, pancreatic, lung, and ovarian cancer: an International Cancer Benchmarking Partnership (ICBP) population-based study. Lancet Oncol. 2024;25:338–51. doi: 10.1016/S1470-2045(24)00031-7. - DOI - PubMed
-
- McPhail S, Barclay ME, Swann R, et al. Use of radiotherapy in patients with oesophageal, stomach, colon, rectal, liver, pancreatic, lung, and ovarian cancer: an International Cancer Benchmarking Partnership (ICBP) population-based study. Lancet Oncol. 2024;25:352–65. doi: 10.1016/S1470-2045(24)00032-9. - DOI - PubMed
-
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–75. doi: 10.1016/S0140-6736(17)33326-3. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
