The NUDIX hydrolase NUDT5 regulates thiopurine metabolism and cytotoxicity
- PMID: 40662362
- PMCID: PMC12259257
- DOI: 10.1172/JCI190443
The NUDIX hydrolase NUDT5 regulates thiopurine metabolism and cytotoxicity
Abstract
Thiopurines are anticancer agents used for the treatment of leukemia and autoimmune diseases. These purine analogs are characterized by a narrow therapeutic index because of the risk of myelosuppression. With the discovery of NUDIX hydrolase 15 (NUDT15) as a major modulator of thiopurine metabolism and toxicity, we sought to comprehensively examine all members of the NUDIX hydrolase family for their effect on the pharmacologic effects of thiopurine. By performing a NUDIX-targeted CRISPR/Cas9 screen in leukemia cells, we identified NUDT5, whose depletion led to drastic thiopurine resistance. NUDT5 deficiency resulted in a nearly complete depletion of active metabolites of thiopurine and the loss of thioguanine incorporation into DNA. Mechanistically, NUDT5 deletion resulted in substantial alteration in purine nucleotide biosynthesis, as determined by steady-state metabolomics profiling. Stable isotope tracing demonstrated that the loss of NUDT5 was linked to a marked suppression of the purine salvage pathway but with minimal effects on purine de novo synthesis. Finally, we comprehensively identified germline genetic variants in NUDT5 associated with thiopurine-induced myelosuppression in 582 children with acute lymphoblastic leukemia. Collectively, these results pointed to NUDT5 as a key regulator of the thiopurine response primarily through its effects on purine homeostasis, highlighting its potential to inform individualized thiopurine therapy.
Keywords: Genetics; Oncology; Pharmacogenetics; Therapeutics.
Figures
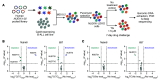
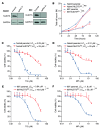
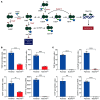


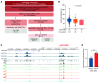
Comment in
- The NUDIX hydrolase NUDT5 influences purine nucleotide metabolism and thiopurine pharmacology
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

