First-generation and preclinical evaluation of an EphA5-targeted antibody-drug conjugate in solid tumors
- PMID: 40662373
- PMCID: PMC12259246
- DOI: 10.1172/JCI188492
First-generation and preclinical evaluation of an EphA5-targeted antibody-drug conjugate in solid tumors
Abstract
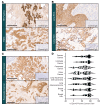
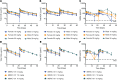
Contemporary cancer treatment strategies are shifting toward targeted therapies to improve efficacy and minimize toxicity. Here, we report the design and preclinical evaluation of MBRC-101, a first-in-class antibody-drug conjugate (ADC) targeting EphA5, a receptor tyrosine kinase with an established role in embryonic development but not extensively studied in cancer. We show that EphA5 is expressed in multiple solid tumors, including cancers of the aerodigestive (non-small cell lung, head and neck, gastric, colon, and pancreatic) and genitourinary (bladder and ovary) tracts, as well as most breast cancer subsets (including triple-negative tumors), with limited expression in normal tissues. MBRC-101 is a humanized anti-EphA5 antibody conjugated to monomethyl auristatin E (MMAE) through a ThioBridge, thereby ensuring stable drug-to-antibody ratio and reducing off-target effects. MBRC-101 showed potent antitumor activity, achieving complete tumor regression in several patient-derived xenograft models. Preclinical Good Laboratory Practice-compliant toxicology studies in rats and nonhuman primates demonstrated that MBRC-101 is well tolerated, with observed toxicities limited to known MMAE off-target effects. These findings establish EphA5 as a therapeutic target in cancer and support the translational development of MBRC-101 as a promising ADC candidate for clinical evaluation, currently in a first-in-human multicenter investigational trial for patients with advanced solid tumors (ClinicalTrials.gov, NCT06014658).
Keywords: Cancer; Cancer immunotherapy; Oncology; Therapeutics.
Conflict of interest statement
Figures