Tomato in the spotlight: light regulation of whole-plant physiology
- PMID: 40662485
- PMCID: PMC12646156
- DOI: 10.1093/jxb/eraf315
Tomato in the spotlight: light regulation of whole-plant physiology
Abstract
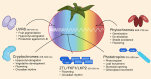
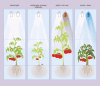
The introduction of light-emitting diodes in plant research and controlled-environment agriculture has given a boost to understanding how light regulates physiology. Here, we review the regulation of whole-plant physiological processes by light in tomato (Solanum lycopersicum), with emphasis on morphogenesis, light interception, photosynthesis, source-sink interactions, assimilate partitioning, fruit set, fruit development, and plant-water relations and how this controls plant growth and fruit quality. Five key aspects of light determine the ultimate plant response, namely intensity, photoperiod, spectrum, directionality, and energy. Tomato possesses five phytochromes, four cryptochromes, two phototropins, one zeitlupe, and one UV-B photoreceptor. Via spectral sensing and photosynthesis, light affects plant morphology, which in turn affects the light interception and consequently whole-plant carbon assimilation. Photosynthesis and carbon partitioning are dynamic processes affected by light. Furthermore, light plays a pivotal role in regulating plant-water-nutrient dynamics by influencing transpiration, stomatal conductance, hydraulic conductance, and cell-wall properties. Changes in light intensity and spectrum can also increase contents of ascorbate, carotenoids, sugars, and volatiles, thereby improving fruit quality. The complex physiological responses of tomato plants to the five aspects of light and their interactions create effectively endless opportunities for future scientific research aimed at improving light-use efficiency, yield, and quality.
Keywords: Solanum lycopersicum; Assimilate partitioning; cryptochrome; fruit quality; morphology; photobiology; photosynthesis; phytochrome; plant–water relations; tomato; transpiration.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Conflict of interest statement
Conflict of interest: The authors declare that they have no conflicts of interest in relation to this work.
Figures



References
-
- Abe H, Yamamoto KT, Nagatani A, Furuya M. 1985. Characterization of green tissue-specific phytochrome isolated immunochemically from pea seedlings. Plant & Cell Physiology 26, 1387–1399.
-
- Affandi FY, Verdonk JC, Ouzounis T, Ji Y, Woltering EJ, Schouten RE. 2020. Far-red light during cultivation induces postharvest cold tolerance in tomato fruit. Postharvest Biology and Technology 159, 111019.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

