Pharmacokinetics and Safety of Polaprezinc Granules Oral Administration in Healthy Chinese Volunteers Under Fasting and Fed Conditions
- PMID: 40663018
- PMCID: PMC12261970
- DOI: 10.1111/cts.70278
Pharmacokinetics and Safety of Polaprezinc Granules Oral Administration in Healthy Chinese Volunteers Under Fasting and Fed Conditions
Abstract
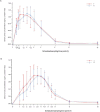
The study aimed to demonstrate bioequivalence between generic and original polaprezinc granules by comparing pharmacokinetic (PK) profiles in healthy Chinese subjects under fasting and fed conditions. This PK investigation was conducted with two independent, randomized, open-label, single-dose, two-period, cross-over studies. Healthy Chinese fasting (N = 24, 75 mg) or fed (N = 24, 300 mg) subjects randomly received a single oral dose of the test or reference polaprezinc granules at each period. Blood samples were collected pre- and post-dose for up to 12 h. Blood zinc was determined using a validated ICP-MS method. Primary PK endpoints were calculated using non-compartmental methods, including peak concentration (Cmax) and the areas under the plasma concentration-time curve (AUC0-t, AUC0-∞). The geometric mean ratios (GMR) in primary PK endpoints between the test and reference products with 90% confidence intervals (CI) were calculated. Treatment-emergent adverse events were assessed. In the fasting study, Cmax, AUC0-t and AUC0-∞ were 1.30 ± 0.30 μg/mL, 4.06 ± 1.13 h·μg/mL, and 4.43 ± 1.04 h·μg/mL following 75 mg test product. In the fed study, Cmax, AUC0-t and AUC0-∞ were 0.91 ± 0.26 μg/mL, 3.26 ± 1.06 h·μg/mL, and 3.37 ± 1.07 h·μg/mL following 300 mg test product. The reference product had comparable PK profiles. All 90% CIs of GMRs in Cmax, AUC0-t and AUC0-∞ between the two products were within 80.0%-125.0%. Both study products were well-tolerated with no serious adverse events. The generic and original polaprezinc granules were bioequivalent by pharmacokinetic comparisons in healthy Chinese subjects under fasting and fed conditions. The two polaprezinc formulations were well-tolerated with no new safety signals. Trial Registration: CTR20210011.
Keywords: bioequivalence; pharmacokinetics; polaprezinc granules; safety; tolerability.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Pharmacokinetics and bioequivalence evaluation of two oral formulations of topiramate tablets in healthy Chinese male volunteers under fasting and fed conditions.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):8619-8627. doi: 10.1007/s00210-024-03782-5. Epub 2025 Jan 21. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39836249 Clinical Trial.
-
Bioequivalence and Safety of Two Amisulpride Formulations in Healthy Chinese Subjects Under Fasting and Fed Conditions: A Randomized, Open‑Label, Single‑Dose, Crossover Study.Drugs R D. 2025 Jun;25(2):117-125. doi: 10.1007/s40268-025-00508-7. Epub 2025 Apr 26. Drugs R D. 2025. PMID: 40287564 Free PMC article. Clinical Trial.
-
Bioequivalence of generic and branded ibrutinib capsules in healthy Chinese volunteers under fasting and fed conditions: a randomized, four-period, fully replicated, crossover study.Expert Opin Drug Metab Toxicol. 2025 Jul;21(7):875-883. doi: 10.1080/17425255.2025.2496459. Epub 2025 Apr 28. Expert Opin Drug Metab Toxicol. 2025. PMID: 40264436 Clinical Trial.
-
Oral budesonide for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2015 Oct 26;2015(10):CD007698. doi: 10.1002/14651858.CD007698.pub3. Cochrane Database Syst Rev. 2015. PMID: 26497719 Free PMC article.
-
Single dose oral ketoprofen or dexketoprofen for acute postoperative pain in adults.Cochrane Database Syst Rev. 2017 May 25;5(5):CD007355. doi: 10.1002/14651858.CD007355.pub3. Cochrane Database Syst Rev. 2017. PMID: 28540716 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

