Evaluating Naphthalene-Modified Metallosalen Complexes as Anticancer Agents
- PMID: 40663054
- PMCID: PMC12305649
- DOI: 10.1021/acs.jmedchem.4c03180
Evaluating Naphthalene-Modified Metallosalen Complexes as Anticancer Agents
Abstract
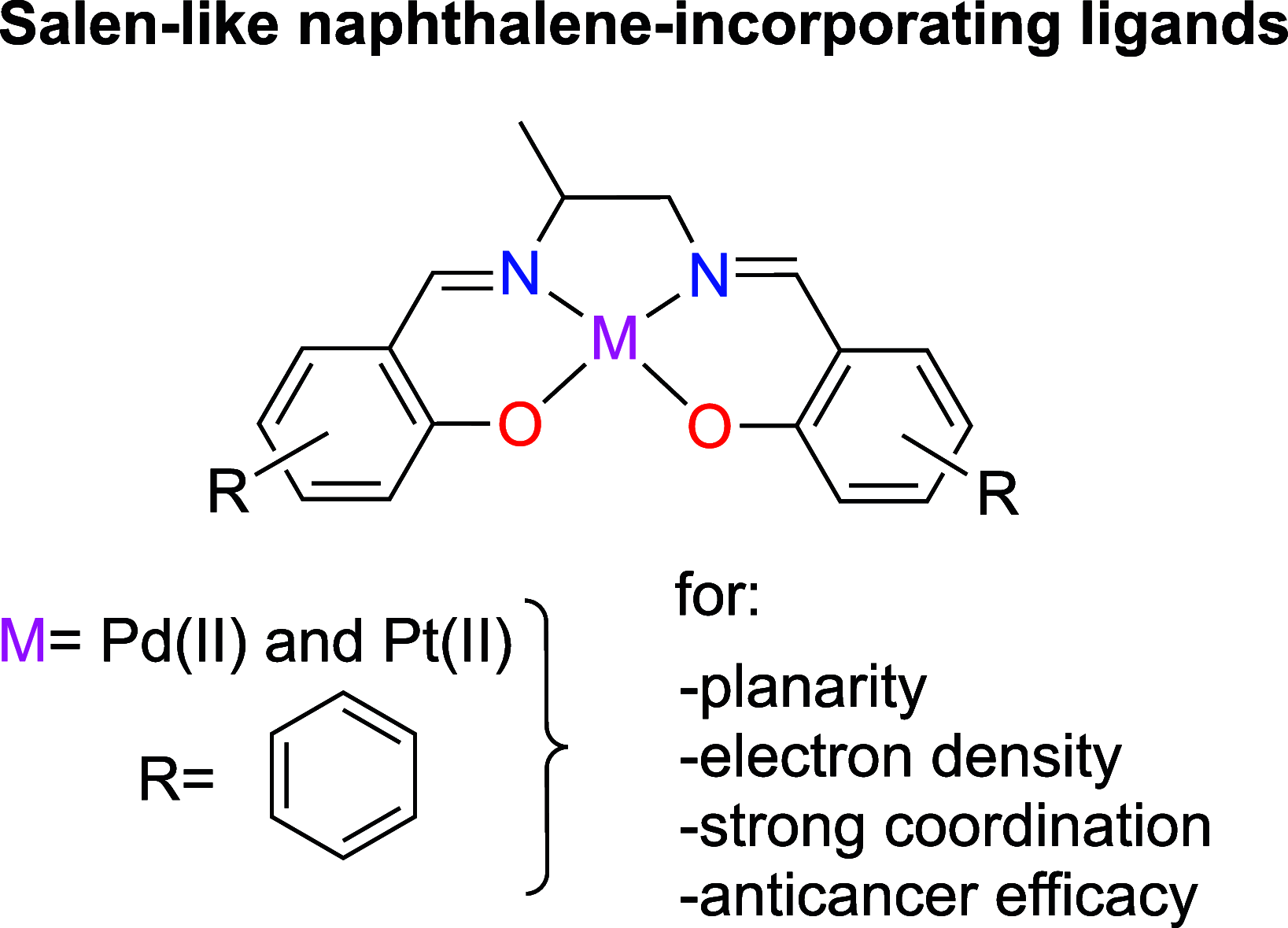
The development of novel naphthalene-modified metallosalens incorporating Pt(II) and Pd(II) presents a promising approach to addressing limitations in cancer therapies. These metallosalens were synthesized and characterized using single crystal X-ray diffraction (sc-XRD), UV-vis spectroscopy, nuclear magnetic resonance (NMR) spectroscopy, and C, H, N elemental analysis. Their strong metal coordination and enhanced electronic interactions, stemming from π-conjugated aromatic structures, improved their DNA-binding affinity and cytotoxic efficacy. Biological assays confirmed significant cytotoxicity in A375 (melanoma) and H292 (nonsmall cell lung cancer) cell lines, while demonstrating minimal toxicity toward HSAEC healthy lung cells. PtL1 and PtL2 exhibited superior activity, inducing apoptosis with high selectivity for cancer cells, as validated by Incucyte Caspase-3/7 Green Dye assays. These findings highlight the potential of naphthalene-modified metallosalens as broad-spectrum cancer therapies, balancing efficacy with reduced off-target effects, and underscore the importance of ligand design and metal coordination in advancing next-generation chemotherapeutics.
Figures
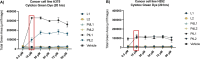




Similar articles
-
Mitochondria-mediated anti-proliferation of triple-negative breast cancer cells by Pd(II)-, Pt(II)-, and Au(III)-NHC complexes of NCN pincers.Dalton Trans. 2025 Jun 24;54(25):10003-10021. doi: 10.1039/d5dt00471c. Dalton Trans. 2025. PMID: 40474752
-
Exploring the antitumor effects of Pd(II) complexes with nitrogen donor ligands towards breast carcinoma.Biometals. 2025 Aug;38(4):1235-1254. doi: 10.1007/s10534-025-00702-9. Epub 2025 Jun 5. Biometals. 2025. PMID: 40471376
-
Enhanced Cytotoxicity of Half-Sandwich Ruthenium(II) Complex Containing Nitro-Substituted Salicyllimidazo[1,5-a]pyridine toward Hormone-Independent Triple-Negative Breast Cancer Cells.Inorg Chem. 2025 Jul 21;64(28):14073-14090. doi: 10.1021/acs.inorgchem.5c00090. Epub 2025 Jul 9. Inorg Chem. 2025. PMID: 40632111
-
The Therapeutic Potential in Cancer of Terpyridine-Based Metal Complexes Featuring Group 11 Elements.ChemMedChem. 2024 May 17;19(10):e202300645. doi: 10.1002/cmdc.202300645. Epub 2024 Feb 28. ChemMedChem. 2024. PMID: 38328860 Review.
-
Exploring the Structure-Activity Relationship of COX Inhibitors with Anticancer Effects: A Comprehensive Review.Curr Top Med Chem. 2025;25(9):1069-1104. doi: 10.2174/0115680266333495241011063253. Curr Top Med Chem. 2025. PMID: 39440774 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

