Metformin promotes osteogenic differentiation of human periodontal ligament stem cells via KLF2-mediated activation of miR-181a-5p under lipopolysaccharide stimulation
- PMID: 40663175
- PMCID: PMC12263736
- DOI: 10.1007/s13577-025-01262-3
Metformin promotes osteogenic differentiation of human periodontal ligament stem cells via KLF2-mediated activation of miR-181a-5p under lipopolysaccharide stimulation
Abstract
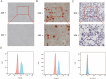
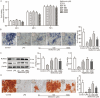
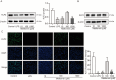
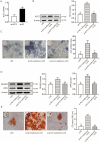
Periodontal ligament stem cells (PDLSCs) constitute a promising source for successful periodontal regeneration. This study aims to explore roles of metformin, krüppel-like factor 2 (KLF2), and miR-181a-5p in mediating osteogenic differentiation of human PDLSCs (hPDLSCs) following lipopolysaccharide (LPS) stimulation. The osteogenic differentiation potential of hPDLSCs isolated from human premolar root samples were examined by alkaline phosphatase (ALP) staining, ALP activity assay, Alizarin red S staining, and Western blotting of osteogenic markers. Metformin pretreatment at dose of 100 μM significantly resulted in increased ALP activity, elevated protein expressions of osteogenic markers, and more generated mineralized matrix in hPDLSCs with LPS stimulation. KLF2 and miR-181a-5p were found to be increased by metformin pretreatment at dose of 100 μM in hPDLSCs with stimulation but not in hPDLSCs without LPS stimulation. The interaction between the KLF2 and the promoter of miR-181a-5p was noted by the dual-luciferase reporter assay. KLF2 knockdown or miR-181a-5p inhibition notably abrogated the improvements of osteogenic differentiation by metformin pretreatment in LPS-stimulated hPDLSCs. The findings of the study indicate metformin protects hPDLSCs against impaired osteogenic differentiation of hPDLSCs after LPS stimulation by KLF2-mediated activation of miR-181a-5p under inflammation conditions.
Keywords: MicroRNA; Periodontitis; Regeneration; Stem cells; Tissue engineering.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: No conflict of interest is declared by the authors. Ethical approval and consent to participation: The study protocol complied with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Changsha Stomatological Hospital. All participants or their guardians had signed the informed consent form prior to recruitment.
Figures

Similar articles
-
KLF2 Promotes Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Regulating Nrf2 Expression.Int Dent J. 2025 Jun;75(3):1554-1563. doi: 10.1016/j.identj.2025.02.019. Epub 2025 Mar 22. Int Dent J. 2025. PMID: 40121852 Free PMC article.
-
PKCβ expression contributes to M1 macrophage-induced impairment in the osteogenic differentiation of periodontal ligament stem cells.In Vitro Cell Dev Biol Anim. 2025 Jun;61(6):635-643. doi: 10.1007/s11626-025-01032-3. Epub 2025 Jun 17. In Vitro Cell Dev Biol Anim. 2025. PMID: 40526258
-
Kaempferol combats the osteogenic differentiation damage of periodontal ligament stem cells in periodontitis via regulating EphrinB2-mediated PI3K/Akt and P38 pathways.Phytomedicine. 2025 Jun;141:156733. doi: 10.1016/j.phymed.2025.156733. Epub 2025 Apr 6. Phytomedicine. 2025. PMID: 40220409
-
M2 macrophage-derived exosome facilitates aerobic glycolysis and osteogenic differentiation of hPDLSCs by regulating TRIM26-induced PKM ubiquitination.Free Radic Biol Med. 2025 Sep;237:88-100. doi: 10.1016/j.freeradbiomed.2025.05.425. Epub 2025 May 29. Free Radic Biol Med. 2025. PMID: 40449810
-
Silencing FOXA1 suppresses inflammation caused by LPS and promotes osteogenic differentiation of periodontal ligament stem cells through the TLR4/MyD88/NF-κB pathway.Biomol Biomed. 2025 Apr 3;25(5):1138-1149. doi: 10.17305/bb.2024.11367. Biomol Biomed. 2025. PMID: 39760528 Free PMC article.
References
-
- Fischer NG, de Souza Araujo IJ, Daghrery A, et al. Guidance on biomaterials for periodontal tissue regeneration: fabrication methods, materials and biological considerations. Dent Mater. 2025;41:283–305. - PubMed
-
- Trubiani O, Pizzicannella J, Caputi S, et al. Periodontal Ligament stem cells: current knowledge and future perspectives. Stem Cells Dev. 2019;28:995–1003. - PubMed
-
- Liang Q, Du L, Zhang R, Kang W, Ge S. Stromal cell-derived factor-1/Exendin-4 cotherapy facilitates the proliferation, migration and osteogenic differentiation of human periodontal ligament stem cells in vitro and promotes periodontal bone regeneration in vivo. Cell Prolif. 2021;54:e12997. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

