'How low can you go?' Developers' perspectives on involving young children in the development of patient reported outcome measures
- PMID: 40663187
- PMCID: PMC12263538
- DOI: 10.1186/s41687-025-00924-y
'How low can you go?' Developers' perspectives on involving young children in the development of patient reported outcome measures
Abstract
Background: Recommendations suggest that children need to be ≥ 8 years-old to participate in concept elicitation (CE) and cognitive interviewing (CI) when developing patient reported outcome measures (PROMs). However, these recommendations have not been subject to thorough scrutiny and recent evidence suggests that younger children may be enabled to participate. This study audited current opinions of PROM developers regarding the feasibility of conducting CE and CI research with children.
Methodology: An online survey was developed to capture PROM developers' perspectives, recruited from existing networks (UK PROMs, International Society for Quality of Life Research) and outcomes research groups from English-speaking countries between August-November 2024. Survey questions explored the ages from which developers considered it feasible to include children in CE and CI research, their previous experiences conducting CE/CI research with children, and respondents' background experiences with children. Results were analysed descriptively, and exploratory comparisons were made based on developers' characteristics.
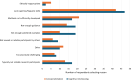
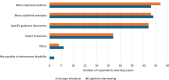
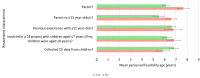
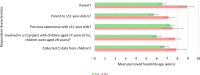
Results: Fifty-eight responses were analysed. The mean youngest ages considered feasible to include children in CE and CI research were 6.66 years and 7.36 years, respectively. The mean youngest ages respondents reported involving children in CE and CI research in practice were 7.67 years and 8.13 years, respectively. Concern that children would have insufficient cognitive and/or linguistic skills was the most often endorsed reason for considering the involvement of younger children to be infeasible. Respondents who had recent parental experience with younger children tended to consider it feasible to include children from younger ages. Those who had conducted CI with children considered it feasible to include children in CI from younger ages. Opposingly, those who had conducted CE with children considered it less feasible to include younger children in CE research.
Conclusions: In-line with established precedent, PROM developers included children from ∼ 8 years-old in CE and CI research, while in principle considering it feasible to include younger ages. Reasons for including (or not including) certain age groups in CE and CI research need critical evaluation and PROM developers may wish to consider ways in which more inclusive opportunities for younger children can be provided.
Keywords: Children; Cognitive interview; Concept elicitation; Patient reported outcome measures; Qualitative research.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Research ethics approval was obtained from the School of Medicine and Population Health Research Ethics Committee at the University of Sheffield (reference number: 063895). Informed consent to participate was obtained via a consent form at the start of the survey; participants could not proceed to complete the survey until consent had been obtained. Consent for publication: Participants were informed that results of the study may be published and that it would not be possible to identify them individually in any way. The authors affirm that participant consent to publish study results was obtained via the consent form presented at the start of the survey. Competing interests: The authors declare that they have no competing interests.
Figures

References
-
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HC (2010) The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol 63(7):737–745. 10.1016/j.jclinepi.2010.02.006 - PubMed
-
- Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, Ring L (2011) Content validity—Establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 2—Assessing respondent Understanding. Value Health 14(8):978–988. 10.1016/j.jval.2011.06.013 - PubMed
-
- Patrick DL, Burke LB, Gwaltney CJ, Leidy NK, Martin ML, Molsen E, Ring L (2011) Content validity—Establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1—Eliciting concepts for a new pro instrument. Value Health 14(8):967–977. 10.1016/j.jval.2011.06.014 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

