Elevated tumor NOS2/COX2 promotes immunosuppressive phenotypes associated with poor survival in ER- breast cancer
- PMID: 40663402
- PMCID: PMC12406732
- DOI: 10.1172/jci.insight.193091
Elevated tumor NOS2/COX2 promotes immunosuppressive phenotypes associated with poor survival in ER- breast cancer
Abstract
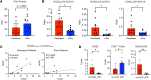
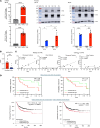
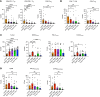
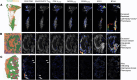
Tumor immunosuppression affects survival and treatment efficacy. Tumor NOS2/COX2 coexpression strongly predicts poor outcome in estrogen receptor-negative (ER-) breast cancer by promoting metastasis, drug resistance, cancer stemness, and immune suppression. Herein, a spatially distinct NOS2/COX2 and CD3+CD8+PD1- T effector (TEff) cell landscape correlated with poor survival in ER- tumors. NOS2 was primarily expressed at the tumor margin, whereas COX2 together with B7H4 was associated with immune desert regions lacking TEff cells, where a higher ratio of tumor NOS2 or COX2 to TEff cells predicted poor survival. Also, programmed cell death ligand 1/programmed cell death 1, regulatory T cells (TRegs), and IDO1 were primarily associated with stroma-restricted TEff cells. Regardless of the survival outcome, CD4+ T cells and macrophages were primarily in stromal lymphoid aggregates. Finally, in a 4T1 model, COX2 inhibition led to increased CD8+ TEff/CD4+ TReg ratio and CD8+ TEff infiltration while Nos2 deficiency had no significant effect, thus reinforcing our observations that COX2 is an essential component of immunosuppression through CD8+ TEff cell exclusion from the tumor. Our study indicates that tumor NOS2/COX2 expression plays a central role in tumor immune evasion, suggesting that strategies combining clinically available NOS2/COX2 inhibitors with immune therapy could provide effective options for the treatment of aggressive and drug-resistant ER- breast tumors.
Keywords: Adaptive immunity; Inflammation; Oncology; T cells.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

