Prophylactic and therapeutic neutralizing monoclonal antibody treatment prevents lethal yellow fever infection
- PMID: 40663406
- PMCID: PMC12416903
- DOI: 10.1172/jci.insight.191665
Prophylactic and therapeutic neutralizing monoclonal antibody treatment prevents lethal yellow fever infection
Abstract
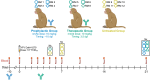
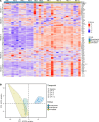
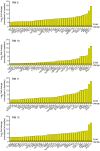
Yellow fever virus (YFV) infection is fatal in 5%-10% of the 200,000 yearly cases. There is currently no available antiviral treatment. We showed previously that administration of 50 mg/kg of a YFV-specific neutralizing monoclonal antibody (nmAb) at 2 days postinfection (dpi), prior to the onset of severe disease, protected YFV-infected rhesus macaques from death. To further explore the clinical applicability of our nmAb MBL-YFV-01, we treated rhesus macaques with a lower dose (10 mg/kg) of this nmAb prophylactically or therapeutically at 3.5 dpi. We show that a single prophylactic or therapeutic i.v. dose of our nmAb protects rhesus macaques from death following challenge. A comprehensive analysis of 167 inflammatory cytokine and chemokines revealed that protection was associated with significantly reduced expression of 125 of these markers, including type I IFN, IL-6, and CCL2. This study further expands the potential clinical use of our YFV-specific nmAb, which could be used during an outbreak for immediate prophylactic immunity or for patients with measurable serum viremia.
Keywords: Hepatitis; Immunology; Infectious disease; Therapeutics.
Figures



References
-
- World Health Organization. Yellow fever. https://www.who.int/news-room/fact-sheets/detail/yellow-fever Accessed July 7, 2025.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

