Efficacy and safety of a therapeutic humanized FSH-blocking antibody in obesity and Alzheimer's disease models
- PMID: 40663423
- PMCID: PMC12404759
- DOI: 10.1172/JCI182702
Efficacy and safety of a therapeutic humanized FSH-blocking antibody in obesity and Alzheimer's disease models
Abstract
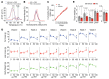
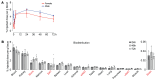
There is growing evidence for direct actions of follicle-stimulating hormone (FSH) on tissues other than the ovaries and testes. Blocking FSH action, either genetically or pharmacologically, protects against bone loss, fat gain, and memory loss in mice. We thus developed a humanized FSH-blocking antibody, MS-Hu6, as a lead therapeutic for 3 diseases of public health magnitude - osteoporosis, obesity, and Alzheimer's disease (AD) - that track together in postmenopausal women. Here, we report the crystal structure of MS-Hu6 and its interaction with FSH in atomistic detail. Using our Good Laboratory Practice platform (21 CFR 58), we formulated MS-Hu6 and the murine equivalent, Hf2, at an ultra-high concentration; both formulated antibodies displayed enhanced thermal and colloidal stability. A single injection of 89Zr-labeled MS-Hu6 revealed a β phase t½ of 79 and 132 hours for female and male mice, respectively, with retention in regions of interest. Female mice injected subcutaneously with Hf2 displayed a dose-dependent reduction in body weight and body fat, in the face of reduced free (bioavailable) FSH and unperturbed estrogen levels. Hf2 also rescued recognition memory and spatial learning loss in a context- and time-dependent manner in AD-prone 3xTg and APP/PS1 mice. MS-Hu6 injected into African green monkeys (8 mg/kg) intravenously, and then subcutaneously at monthly intervals, was safe, and without effects on vital signs, blood chemistries, or blood counts. There was a notable approximately 4% weight loss in all 4 monkeys after the first injection, which continued in 2 of the monkeys. We thus provide Investigational New Drug-enabling data for a planned first-in-human study.
Keywords: Adipose tissue; Drug therapy; Endocrinology; Neurodegeneration; Therapeutics.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

