Comprehensive analysis of human keratinocyte interactions with Candida albicans and Candida parapsilosis
- PMID: 40663456
- PMCID: PMC12323423
- DOI: 10.1080/21505594.2025.2532815
Comprehensive analysis of human keratinocyte interactions with Candida albicans and Candida parapsilosis
Abstract
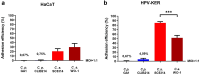
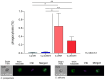
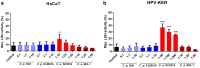
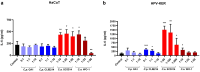
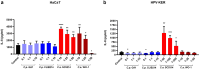
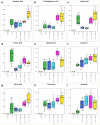
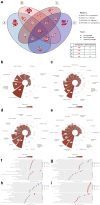
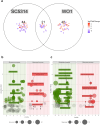
In recent years, microbiome studies have revealed that Candida species are common colonizers of the human skin. The distribution of species, however, varies greatly. Although C. parapsilosis is more likely to resemble skin commensals, opinions are divided, and discrepancies are present regarding C. albicans that is also often associated with cutaneous candidiasis. Therefore, we aimed to thoroughly assess the nature of skin epithelial cell - Candida interactions. To study species-specific host responses, we examined internalization, cytokine and metabolic responses in different keratinocytes (HaCaT, HPV-KER) along with host cell damage following fungal stimuli. To rigorously examine yeast-keratinocyte interactions, we applied two distinct isolates of both C. albicans (SC5314, WO-1) and C. parapsilosis (GA1, CLIB214). Comparison of the two fungi's virulence revealed that while C. albicans effectively adheres to human keratinocytes and causes subsequent damage, C. parapsilosis is unable to establish lasting physical contact and causes less harm. In terms of keratinocyte response, both cell lines showed significantly enhanced cellular (internalization), humoral (IL-6, IL-8) and metabolic responses (2-ketoglutaric acid, citric acid, threorine, hypotaurine) to C. albicans strains, while those towards C. parapsilosis remained relatively low or similar to the control condition. Under certain conditions strain preference was also detected. Of the two cell lines, HPV-KER was more sensitive, as besides interspecies differences, intraspecies differences were also measurable. These results suggest that C. albicans triggers an enhanced antifungal response, thus does not closely resemble skin commensals, like C. parapsilosis. Furthermore, HPV-KER might serve as a more applicable tool for studying keratinocyte antifungal responses.
Keywords: Keratinocyte; candida; immune response; interaction; pathogenic fungi.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Histone deacetylase Sir2 promotes the systemic Candida albicans infection by facilitating its immune escape via remodeling the cell wall and maintaining the metabolic activity.mBio. 2024 Jun 12;15(6):e0044524. doi: 10.1128/mbio.00445-24. Epub 2024 Apr 29. mBio. 2024. PMID: 38682948 Free PMC article.
-
Epidemiology and antifungal susceptibility of fungal infections from 2018 to 2021 in Shandong, eastern China: A report from the SPARSS program.Indian J Med Microbiol. 2024 Jan-Feb;47:100518. doi: 10.1016/j.ijmmb.2023.100518. Epub 2023 Dec 5. Indian J Med Microbiol. 2024. PMID: 38016503
-
Transcriptomic insights into Candida albicans adaptation to an anaerobic environment.Microbiol Spectr. 2025 Jul;13(7):e0302424. doi: 10.1128/spectrum.03024-24. Epub 2025 May 22. Microbiol Spectr. 2025. PMID: 40401963 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
