A genetically defined pontine nucleus essential for ingestion in mice
- PMID: 40663610
- PMCID: PMC12305073
- DOI: 10.1073/pnas.2411174122
A genetically defined pontine nucleus essential for ingestion in mice
Abstract
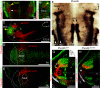
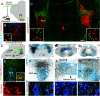
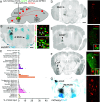
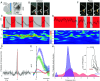
The first phase of feeding consists in the procurement of solid foods from the environment by biting, and their preparation for swallowing by chewing. These actions require the precise coordination of tens of orofacial muscles for the jaw and tongue. The seat for this motor patterning is known to reside in the reticular formation, a complex and poorly mapped region of the hindbrain, but the neuron groups involved are still elusive. Here, we characterize a group of excitatory reticular interneurons located in the supratrigeminal area that express the homeodomain transcription factor Phox2b. This nucleus-Sup5Phox2b-is premotor to both jaw-closing and jaw-opening motoneurons and receives direct input from cranial sensory afferents, motor cortex, and satiation related nuclei. Its activity differentially tracks lapping, biting, and chewing movements, suggesting its involvement in the elaboration of distinct orofacial motor patterns in vivo. Acute global activation or inhibition of Sup5Phox2b by optogenetics interrupt volitional feeding sequences. Thus, Sup5Phox2b is an obligatory subcortical node, topologically and genetically defined, in the neural circuits that control the oral phase of feeding in mice.
Keywords: brainstem; feeding; motor systems; orofacial movements; systems neuroscience.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Comment in
-
Into the belly of the brainstem: The neurons of the "extended" enteric nervous system.Proc Natl Acad Sci U S A. 2025 Aug 26;122(34):e2516545122. doi: 10.1073/pnas.2516545122. Epub 2025 Aug 18. Proc Natl Acad Sci U S A. 2025. PMID: 40825137 No abstract available.
Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Peripuberty Is a Sensitive Period for Prefrontal Parvalbumin Interneuron Activity to Impact Adult Cognitive Flexibility.Dev Neurosci. 2025;47(2):127-138. doi: 10.1159/000539584. Epub 2024 Jun 3. Dev Neurosci. 2025. PMID: 38830346 Free PMC article.
-
Pathological laughter and crying: insights from lesion network-symptom-mapping.Brain. 2021 Nov 29;144(10):3264-3276. doi: 10.1093/brain/awab224. Brain. 2021. PMID: 34142117 Review.
-
Interventions for dysphagia in long-term, progressive muscle disease.Cochrane Database Syst Rev. 2016 Feb 9;2(2):CD004303. doi: 10.1002/14651858.CD004303.pub4. Cochrane Database Syst Rev. 2016. PMID: 26859621 Free PMC article.
References
-
- Yoshida Y., Miyazaki T., Hirano M., Shin T., Kanaseki T., Topographic arrangement of motoneurons innervating the suprahyoid and infrahyoid muscles. A horseradish peroxidase study in cats. Ann. Otol. Rhinol. Laryngol. 92, 259–266 (1983). - PubMed
-
- Grill H. J., Norgren R., The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 143, 281–297 (1978). - PubMed
-
- Lund J. P., Dellow P. G., The influence of interactive stimuli on rhythmical masticatory movements in rabbits. Arch. Oral Biol. 16, 215–223 (1971). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

