Gabapentin for Pain Management after Major Surgery: A Placebo-controlled, Double-blinded, Randomized Clinical Trial (the GAP Study)
- PMID: 40663783
- PMCID: PMC12416896
- DOI: 10.1097/ALN.0000000000005655
Gabapentin for Pain Management after Major Surgery: A Placebo-controlled, Double-blinded, Randomized Clinical Trial (the GAP Study)
Abstract
Background: Gabapentin is an anticonvulsant medication with approval for use in neuropathic pain and epileptic disorders. It is frequently added to multimodal analgesic regimens during and after surgery to reduce opioid use while controlling pain effectively. There is little evidence to show its effectiveness in major surgery.
Methods: In this multicenter, double-blinded randomized controlled trial, adults undergoing major cardiac, thoracic, or abdominal surgery were randomized to receive either gabapentin (600 mg before surgery, 300 mg twice daily for 2 days after surgery) or placebo. The primary outcome was length of hospital stay. Secondary outcomes included acute and chronic pain, total opioid use, adverse health events, and health-related quality of life. Patients were followed up daily in-hospital until discharge and then at 4 weeks and 4 months after surgery.
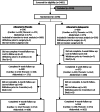
Results: A total of 1,196 participants were randomized (500 underwent cardiac, 346 thoracic, and 350 abdominal surgery); 596 were allocated to placebo, and 600 were allocated to gabapentin. Median length of hospital stay was similar in the two groups (gabapentin, 5.94 [interquartile range (IQR), 4.08 to 8.04] days; placebo, 6.15 [IQR, 4.22 to 8.97] days; hazard ratio, 1.07; 95% CI, 0.95 to 1.20; P = 0.26). Overall, 384 participants experienced one or more serious adverse events (gabapentin, 189 of 596 [31.7%]; placebo, 195 of 599 [32.6%]), with some variation across surgical specialties.
Conclusions: Among patients undergoing major cardiac, thoracic, and abdominal surgery, adding gabapentin to multimodal analgesic regimes did not alter the length of hospital stay or the number of serious adverse events.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc., on behalf of the American Society of Anesthesiologists.
Conflict of interest statement
Dr. Batchelor has received speaker fees/advisory board fees from JnJ, Medtronic, and Bristol Myers Squibb. Dr. Edwards has received an honorariam for a lecture from Edwards Lifesciences. Dr. Grocott declares the following conflicts of interest: Edwards Lifesciences (consultancy/medical advisory board). Dr. Casali left employment at the institution at which the study was being conducted during the trial; he is now employed by JnJ Med Tech. The other authors declare no competing interests.
The article processing charge was funded by the United Kingdom National Institute of Health and Care Research.
Figures



References
-
- Dooley DJ, Taylor CP, Donevan S, Feltner D: Ca2+ channel α2δ ligands: Novel modulators of neurotransmission. Trends Pharmacol Sci 2007; 28:75–82. doi:10.1016/j.tips.2006.12.006 - PubMed
-
- Taylor CP, Gee NS, Su T-Z, et al. : A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res 1998; 29:233–49. doi:10.1016/s0920-1211(97)00084-3 - PubMed
-
- Martinez V, Carles M, Marret E, Beloeil H: Perioperative use of gabapentinoids in France: Mismatch between clinical practice and scientific evidence. Anaesth Crit Care Pain Med 2018; 37:43–7. doi:10.1016/j.accpm.2017.01.010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

