Clinical evaluation of the novel digital liquid chip method for anti-dsDNA detection in SLE
- PMID: 40664440
- PMCID: PMC12265827
- DOI: 10.1136/lupus-2025-001608
Clinical evaluation of the novel digital liquid chip method for anti-dsDNA detection in SLE
Abstract
Objective: This study aims to evaluate the diagnostic performance of the digital liquid chip method (DLCM) compared with indirect immunofluorescence (IIF) and chemiluminescent immunoassay (CLIA) for anti-double-stranded DNA (dsDNA) antibody detection in SLE.
Methods: The retrospective study consecutively enrolled 1349 patients, including 698 with SLE and 651 with other autoimmune diseases at Peking Union Medical College Hospital. Anti-dsDNA antibodies were detected using IIF (EUROIMMUN, Luebeck, Germany), CLIA (YHLO, Shenzhen, China) and DLCM (Livzon, Zhuhai, China). The sensitivity, specificity and area under the curve (AUC) of each method and combination were compared at the recommended manufacturer cut-offs. The agreement between methods and the association between antibody levels and clinical characteristics including disease activity, complement levels and organ involvement were also evaluated.
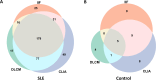
Results: All methods exhibited high specificity, while IIF performed best (98.5%), significantly greater than CLIA (96.3%) and DLCM (96.6%) (p < 0.05). CLIA demonstrated the highest sensitivity (48.1%), outperforming IIF (36.0%) and DLCM (41.4%) (p<0.001). Cohen's kappa indicated substantial positive agreement between DLCM and CLIA (κ=0.67), and moderate agreement between IIF and the other methods (κ=0.52-0.55). Combining IIF with DLCM or CLIA improved diagnosis performance, with IIF+CLIA achieving the highest sensitivity (54.0%), accuracy (74.1%) and AUC (0.75). Moreover, anti-dsDNA positivity was strongly associated with lower complement levels (C3: 0.71 vs 0.90 g/L in DLCM+ vs DLCM-, p<0.001) and moderate-severe disease activity (65.0% DLCM positive). DLCM uniquely predicted musculoskeletal involvement (55.3% vs 44.7%, p<0.01). However, the diagnostic performance for renal involvement was limited (sensitivity 46.9%, specificity 56.3%, AUC=0.52).
Conclusions: DLCM demonstrated substantial agreement with CLIA and held potential for SLE diagnosis and monitoring. A multiassay strategy, using a sensitive assay like CLIA or DLCM for initial screening and a highly specific assay like IIF for confirmation, optimises diagnostic performance for anti-dsDNA antibody detection in SLE.
Keywords: Autoantibodies; Autoimmune Diseases; Sensitivity and Specificity; Systemic Lupus Erythematosus.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

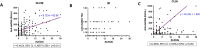
Similar articles
-
Comparative evaluation of three anti-dsDNA antibody detection methods in systemic lupus erythematosus: insights from a large monocentric cohort.Front Immunol. 2025 Apr 10;16:1529484. doi: 10.3389/fimmu.2025.1529484. eCollection 2025. Front Immunol. 2025. PMID: 40276498 Free PMC article.
-
Evaluating the performance of four anti-dsDNA antibody detection methods.Lupus. 2025 Aug 23:9612033251371116. doi: 10.1177/09612033251371116. Online ahead of print. Lupus. 2025. PMID: 40848232
-
Comparison of four methods of detection of anti-double-stranded DNA in SLE patients.Egypt J Immunol. 2025 Jul;32(3):59-65. doi: 10.55133/eji.320307. Egypt J Immunol. 2025. PMID: 40684354
-
Are anti-nucleosome antibodies a better diagnostic marker than anti-dsDNA antibodies for systemic lupus erythematosus? A systematic review and a study of metanalysis.Autoimmun Rev. 2012 Dec;12(2):97-106. doi: 10.1016/j.autrev.2012.07.002. Epub 2012 Jul 15. Autoimmun Rev. 2012. PMID: 22810055
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD013652. doi: 10.1002/14651858.CD013652.pub2. Cochrane Database Syst Rev. 2022. PMID: 36394900 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
