Randomized phase II study of consolidation immunotherapy with nivolumab and ipilimumab or nivolumab alone following concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non-small-cell lung cancer (NSCLC): Big Ten Cancer Research Consortium LUN16-081
- PMID: 40664454
- PMCID: PMC12265819
- DOI: 10.1136/jitc-2024-010316
Randomized phase II study of consolidation immunotherapy with nivolumab and ipilimumab or nivolumab alone following concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non-small-cell lung cancer (NSCLC): Big Ten Cancer Research Consortium LUN16-081
Abstract
Background: For unresectable stage III non-small-cell lung cancer (NSCLC), the optimal duration and regimen of consolidation immunotherapy following chemoradiation is unknown. Despite improved outcomes with 12 months of durvalumab, which has become the standard of care, new strategies to improve survival are needed. This study evaluates dual immunotherapy in the consolidation setting following concurrent chemoradiation as well as a shorter (6 months) treatment duration.
Methods: Following concurrent chemoradiation, subjects were randomized 1:1 to nivolumab alone (480 mg intravenously every 4 weeks) or combination nivolumab (240 mg intravenously every 2 weeks) and ipilimumab (1 mg/kg every 6 weeks) for up to 6 months. Primary endpoint was 18-month progression-free survival (PFS), and each arm was compared with appropriate historical controls. Secondary endpoints included overall survival (OS), time to metastatic disease, and toxicity.
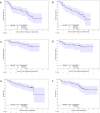
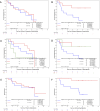
Results: 105 patients enrolled (54 nivolumab alone; 51 nivolumab/ipilimumab). Median follow-up was 29.1 months for nivolumab and 30 months for nivolumab/ipilimumab. For nivolumab alone, 18-month PFS was 65.5% (95% CI, 49.8% to 77.3%), a statistically significant improvement over the historical control of chemoradiation alone (p<0.1). Median PFS was 29.7 months (95% CI, 16.5 to Not Reached), and median OS was 32 months (95% CI, 32 to NR). For nivolumab/ipilimumab, 18-month PFS was 66.3% (95% CI, 56.3% to 74.5%), a statistically significant improvement over the historical control of chemoradiation followed by durvalumab (p<0.1). Median PFS was 26.3 months (95% CI, 18.6 to NR), and median OS was not reached (95% CI, 30.9 to NR). Rate of any-grade treatment-related adverse events was 72.2% (grade ≥3=18.5%) for nivolumab and 80.4% (grade ≥3=29.4%) for nivolumab/ipilimumab. Most common adverse events (grade 3/4) for nivolumab were fatigue 31.5% (0%), pneumonitis 20.4% (7.4%), rash 16.7% (3.7%), dyspnea 14.8% (0%), and hypothyroidism 14.8% (0%). For nivolumab/ipilimumab, they were fatigue 31.4% (3.9%), pneumonitis 23.5% (11.7%), diarrhea 19.6% (2%), dyspnea 19.6% (0%), pruritus 17.7% (0%), hypothyroidism 15.7% (0%), rash 15.7% (2%), arthralgias 11.8% (0%), and nausea 11.8% (0%). Grade 2 or higher pneumonitis was similar for nivolumab (20.4%) and nivolumab/ipilimumab (19.6%), but grade 3 or higher pneumonitis was more common in the nivolumab/ipilimumab arm (11.8% vs 7.4%).
Conclusion: Despite only 6 months of consolidation immunotherapy, nivolumab alone and combination nivolumab/ipilimumab both demonstrated improved 18-month PFS over appropriate historical controls. Overall toxicity, including grade 3 pneumonitis, was higher in the combination arm.
Keywords: Ipilimumab; Lung Cancer; Nivolumab; Pneumonitis.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: Several authors do receive grant support, honoraria, or consulting fees from Bristol Myers or other entities with interest in immuno-oncology, but I can attest that these relationships have not conflicted the conduct or analysis of this study in any way. Full COI disclosure forms can be submitted on request.
Figures
References
-
- Durm GA, Jabbour SK, Althouse SK, et al. A phase 2 trial of consolidation pembrolizumab following concurrent chemoradiation for patients with unresectable stage III non-small cell lung cancer: Hoosier Cancer Research Network LUN 14-179. Cancer. 2020;126:4353–61. doi: 10.1002/cncr.33083. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
