Reinforced Odor Representations in the Anterior Olfactory Nucleus Can Serve as Memory Traces for Conspecifics
- PMID: 40664512
- PMCID: PMC12303586
- DOI: 10.1523/ENEURO.0143-25.2025
Reinforced Odor Representations in the Anterior Olfactory Nucleus Can Serve as Memory Traces for Conspecifics
Abstract
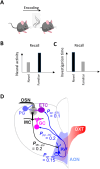
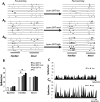
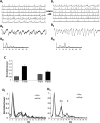
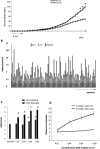
Recognition of conspecific individuals in mammals is an important skill, thought to be mediated by a distributed array of neural networks, including those processing olfactory cues. Recent data from our groups have shown that social memory can be supported by olfactory cues alone and that interactions with an individual lead to increased neural representations of that individual in the anterior olfactory nucleus, an olfactory network strongly modulated by the neuropeptide oxytocin. We here show, using a computational model, how enhanced representations in the AON can easily arise during the encoding phase, how they can be modulated by OXT, and how a dynamic memory signature in the form of enhanced oscillations in the beta range arises from the architecture of the neural networks involved. These findings have implications for our understanding how social memories are formed and retrieved and generate further hypotheses that can be tested experimentally.
Keywords: computation; conspecific; learning; memory; olfactory; oxytocin.
Copyright © 2025 Linster et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Q-learning with temporal memory to navigate turbulence.Elife. 2025 Jul 21;13:RP102906. doi: 10.7554/eLife.102906. Elife. 2025. PMID: 40690282 Free PMC article.
-
In mice, discrete odors can selectively promote the neurogenesis of sensory neuron subtypes that they stimulate.Elife. 2025 Jun 18;13:RP96152. doi: 10.7554/eLife.96152. Elife. 2025. PMID: 40531183 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
The Lived Experience of Autistic Adults in Employment: A Systematic Search and Synthesis.Autism Adulthood. 2024 Dec 2;6(4):495-509. doi: 10.1089/aut.2022.0114. eCollection 2024 Dec. Autism Adulthood. 2024. PMID: 40018061 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
