Disrupted Neuronal Dynamics of Reward Encoding in the Medial Prefrontal Cortex and the Ventral Tegmental Area after Episodic Social Stress
- PMID: 40664513
- PMCID: PMC12309445
- DOI: 10.1523/ENEURO.0229-25.2025
Disrupted Neuronal Dynamics of Reward Encoding in the Medial Prefrontal Cortex and the Ventral Tegmental Area after Episodic Social Stress
Abstract
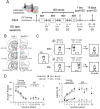
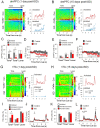
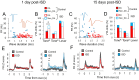
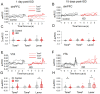
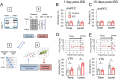
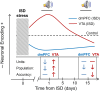
Previous research suggests that stress predisposes individuals to develop substance use disorders by disrupting the brain processing of rewards. Yet, how stressful experiences disrupt the brain processing of reward-related cues at the neuronal level is poorly understood. Intermittent social defeat (ISD) is a stress animal model that increases reward-seeking behavior, drug self-administration, and choice impulsivity up to several weeks after stress. We tested the hypothesis that ISD disrupts the neuronal encoding of reward cues in key areas of the brain that regulate reward-seeking. We examined in vivo neuronal dynamics in response to reward cues in the dorsal medial prefrontal cortex (dmPFC) and the ventral tegmental area (VTA) simultaneously, and longitudinally, in control and stressed Long-Evans male rats during a discriminative stimulus reward-seeking task. In the dmPFC, ISD decreased cue-evoked neuronal activity 1 and 15 d after stress, which indicates a long-term degradation of outcome anticipation-related processing. In the VTA, ISD increased cue-evoked neuronal activity 1 d after stress but decreased cue-evoked activity 15 d after stress. Moreover, decoding analysis in single neurons and populations showed parallel increases and decreases in reward discrimination accuracy in the VTA which points to time-dependent changes in incentive salience after stress. These results demonstrate that ISD differently disrupts the neuronal encoding of reward cues in the dmPFC and the VTA and identify novel neurofunctional signatures that underlie a higher predisposition to seek out rewards after stress.
Keywords: neuronal population; rat; reward-seeking behavior; single neurons; sustained activity; vulnerability.
Copyright © 2025 Harris et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Ventral tegmental area dopamine neural activity switches simultaneously with rule representations in the medial prefrontal cortex and hippocampus.J Neurosci. 2025 Mar 17:e1670242025. doi: 10.1523/JNEUROSCI.1670-24.2025. Online ahead of print. J Neurosci. 2025. PMID: 40097186
-
Modulation of Dopamine Neurons Alters Behavior and Event Encoding in the Nucleus Accumbens during Pavlovian Conditioning.J Neurosci. 2025 Jun 25;45(26):e0061252025. doi: 10.1523/JNEUROSCI.0061-25.2025. J Neurosci. 2025. PMID: 40393804
-
Involvement of a stress-responsive orbito-striatal projection in impulsive action in male rats.Int J Neuropsychopharmacol. 2025 Jul 23;28(7):pyaf043. doi: 10.1093/ijnp/pyaf043. Int J Neuropsychopharmacol. 2025. PMID: 40576337 Free PMC article.
-
Social Support Effects on Neural Stress and Alcohol Reward Responses.Curr Top Behav Neurosci. 2022;54:461-482. doi: 10.1007/7854_2021_246. Curr Top Behav Neurosci. 2022. PMID: 34734391 Review.
-
Leveraging individual differences in cue-reward learning to investigate the psychological and neural basis of shared psychiatric symptomatology: The sign-tracker/goal-tracker model.Behav Neurosci. 2024 Aug;138(4):260-271. doi: 10.1037/bne0000590. Epub 2024 May 16. Behav Neurosci. 2024. PMID: 38753398 Free PMC article. Review.
References
-
- Akalin A (2020) Computational genomics with R, Ed 1. New York: CRC Press LLC.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
