Publication of data sharing statements in clinical trials by cardiovascular journals: a quantitative and qualitative analysis
- PMID: 40664672
- PMCID: PMC12263965
- DOI: 10.1038/s41597-025-05510-x
Publication of data sharing statements in clinical trials by cardiovascular journals: a quantitative and qualitative analysis
Abstract
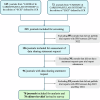
Many cardiovascular disease (CVD) journals request data sharing statements upon trial report submission, but their compliance in publishing these statements remains unclear. We therefore performed a quantitative analysis to evaluate the current practice of the publications of data sharing statements in clinical trials by CVD journals, which included 78 CVD journals that published clinical trials from Jan 2019 to Dec 2022 and had data sharing statement request. Multivariable logistic regression analysis was used to examine the association between journal characteristics and journals' publications of statements. We also ran an online qualitative survey by sending anonymous questionnaires to editors-in-chief from CVD journals for their opinions on journals' publications of statements, trying to further explore why the journals did not publish statements. Their perspectives could provide in-depth information on and new insights into promoting publications of data sharing statements. This quantitative and qualitative analysis assessing the current practice of publishing data sharing statements by CVD journals, may generate new evidence to promote the actual data sharing and transparency in CVD trials.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: G.Y.H.L. has served as a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, and Daiichi-Sankyo. No fees have been received directly or personally. All other authors have declared no conflicts of interest.
Figures


Similar articles
-
Data sharing statements for clinical trials: a cross-sectional survey of cardiology journals.BMJ Open. 2025 Jul 22;15(7):e097148. doi: 10.1136/bmjopen-2024-097148. BMJ Open. 2025. PMID: 40701601 Free PMC article.
-
Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals.Cochrane Database Syst Rev. 2012 Nov 14;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152285 Free PMC article.
-
Do peer reviewers comment on reporting items as instructed by the journal? A secondary analysis of two randomized trials.J Clin Epidemiol. 2025 Jul;183:111818. doi: 10.1016/j.jclinepi.2025.111818. Epub 2025 May 8. J Clin Epidemiol. 2025. PMID: 40348145
-
Assessment of whether use of a professional medical writer is associated with time to publication of papers reporting phase III oncology clinical trials in two high-impact general medicine journals.Curr Med Res Opin. 2025 May;41(5):887-893. doi: 10.1080/03007995.2025.2505697. Epub 2025 May 23. Curr Med Res Opin. 2025. PMID: 40372172
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
References
-
- Taichman, D. B. et al. Sharing Clinical Trial Data: A Proposal From the International Committee of Medical Journal Editors. JAMA315(5), 467–468 (2016). - PubMed
-
- Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021. Lancet403(10440), 2133–2161 (2024). - PMC - PubMed
-
- Bauchner, H., Golub, R. M. & Fontanarosa, P. B. Data Sharing: An Ethical and Scientific Imperative. JAMA315(12), 1237–1239 (2016). - PubMed
-
- Warren, E. Strengthening Research through Data Sharing. N Engl J Med375(5), 401–403 (2016). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

