Exo-miR-1911-5p regulates ferroptosis to promote macrophages M2 polarization-mediated gastric cancer cisplatin resistance via MYB/AKR1B10/ACC
- PMID: 40664751
- PMCID: PMC12263902
- DOI: 10.1038/s42003-025-08441-w
Exo-miR-1911-5p regulates ferroptosis to promote macrophages M2 polarization-mediated gastric cancer cisplatin resistance via MYB/AKR1B10/ACC
Abstract
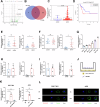
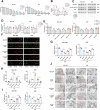
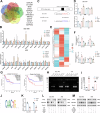
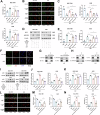
Tumor-associated macrophages (TAMs) have been implicated in fostering various hallmarks of cancer progression in gastric cancer (GC). However, the intricate molecular mechanisms underlying TAM-induced chemoresistance remain incompletely understood. Exosomes emerge as key players, mediating TAM-induced resistance to cisplatin (DDP) by regulating ferroptosis. Our investigation reveals that exo-miR-1911-5p, delivered to GC cells from TAMs, significantly contributes to cisplatin resistance. Specifically, direct modulation of MYB by MiR-1911-5p leads to decreased expression of AKR1B10, a crucial factor in preventing ferroptosis. Further exploration confirms the regulation of ACC by AKR1B10. Through targeting the MYB/AKR1B10/ACC axis, exo-miR-1911-5p inhibits ferroptosis to enhances cisplatin resistance. Additionally, exo-miR-1911-5p promotes M2 polarization of TAMs by targeting ARHGEF3. Collectively, our findings highlight the critical role of exo-miR-1911-5p in mediating cisplatin resistance through modulating the cross-talk between TAMs and GC. Targeting exo-miR-1911-5p could represent a promising strategy for overcoming DDP resistance in GC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249 (2021). - PubMed
-
- Van Cutsem, E., Sagaert, X., Topal, B., Haustermans, K. & Prenen, H. Gastric cancer. Lancet388, 2654–2664 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

