Fatigue in long-term cancer survivors: prevalence, associated factors, and mortality. A prospective population-based study
- PMID: 40665014
- PMCID: PMC12449452
- DOI: 10.1038/s41416-025-03116-z
Fatigue in long-term cancer survivors: prevalence, associated factors, and mortality. A prospective population-based study
Abstract
Background: We compared fatigue severity in breast, prostate or colorectal cancer survivors 5-16 years post-diagnosis with cancer-free controls, and examined factors associated with fatigue and its association with all-cause mortality in survivors.
Methods: Participants of the CAncEr Survivorship - A multi-Regional (CAESAR) study completed the Fatigue Assessment Questionnaire (FAQ) between 2009 and 2011. The FAQ assesses affective, cognitive, and physical fatigue, and sleep problems. We derived the odds of fatigue using logistic regression with the 75th percentile of population norms as the cut-off. All-cause mortality (up to end 2021) was estimated using Cox regression models.
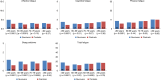
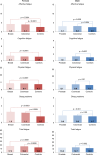
Results: The sample comprised 6057 survivors, of whom approximately one-third reported affective, cognitive, or physical fatigue. Demographic (age, relationship), clinical (chemotherapy), comorbidity (depression), lifestyle, and psychological factors were associated with higher odds of fatigue symptoms and total fatigue. Fatigue symptoms, predominantly physical fatigue, were strongly associated with mortality (unadjusted hazard ratios (HRs) ranged from 1.48 to 2.40). The HRs were attenuated after adjustment for comorbidities and depressive symptoms, although affective and physical fatigue remained independent risk factors for mortality.
Conclusions: Demographic, clinical, comorbidity, lifestyle, and psychological factors were associated with fatigue in long-term survivors. Fatigued survivors have a higher mortality risk. Lowering the burden of fatigue by a comprehensive approach might result in better survival.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The ethics committee of the University of Heidelberg (S499/2012) and the responsible local ethics committees of the participating cancer registries approved the CAESAR study. All participants provided written informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Figures
References
-
- De Angelis R, Demuru E, Baili P, Troussard X, Katalinic A, Chirlaque Lopez MD, et al. Complete cancer prevalence in Europe in 2020 by disease duration and country (EUROCARE-6): a population-based study. Lancet Oncol. 10.1016/S1470-2045(23)00646-0 (2024). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

