Efficacy and Safety of Lebrikizumab in Adult and Adolescent Patients with Skin of Color and Moderate-to-Severe Atopic Dermatitis: Results from the Phase IIIb, Open-Label ADmirable Study
- PMID: 40665146
- PMCID: PMC12436573
- DOI: 10.1007/s40257-025-00970-8
Efficacy and Safety of Lebrikizumab in Adult and Adolescent Patients with Skin of Color and Moderate-to-Severe Atopic Dermatitis: Results from the Phase IIIb, Open-Label ADmirable Study
Abstract
Background: Data are lacking to guide diagnosis and treatment in patients with skin of color and atopic dermatitis (AD), a population traditionally underrepresented in clinical trials.
Objective: The aim of this study was to evaluate the efficacy and safety of lebrikizumab in adults and adolescents with skin of color and moderate-to-severe AD.
Methods: In the open-label ADmirable trial, 90 adults and adolescents with moderate-to-severe AD, Fitzpatrick skin phototype IV-VI, and self-reported race other than White received lebrikizumab 250 mg subcutaneously every 2 weeks (Q2W), following a 500-mg loading dose at baseline and Week 2, for 16 weeks. From Week 16 to Week 24, responders, defined as patients with at least 75% improvement in Eczema Area and Severity Index (EASI 75) and/or Investigator's Global Assessment (IGA) score of 0/1 with at least a 2-point improvement from baseline, received lebrikizumab every 4 weeks (Q4W); inadequate responders continued lebrikizumab Q2W. The primary endpoint was the percentage of patients achieving EASI 75 at Week 16. Secondary and exploratory efficacy endpoints and safety were assessed throughout. Data were analyzed as observed and using imputation, with Q2W and Q4W populations pooled for Weeks 16-24.
Results: Mean age at baseline was 40.7 years; 43.3% were female; and 43.3%, 24.4%, and 32.2% had Fitzpatrick skin phototypes IV, V, and VI, respectively. Baseline mean EASI and Pruritus Numeric Rating Scale (NRS) scores were 26.4 and 7.0, respectively; 68.9% of patients had moderate disease (IGA = 3). At Week 16 (number of patients with non-missing values [Nx] = 78), EASI 75, EASI 90 (≥ 90% improvement from baseline in EASI), and IGA 0/1 (IGA response of clear or almost clear) were achieved by 69.2%, 44.9%, and 44.9% of patients, respectively; for Pruritus NRS (Nx = 62), 58.1% of patients reported ≥ 4-point improvement. At Week 24 (Nx = 74) (pooled treatment arms), EASI 75, EASI 90, and IGA 0/1 were achieved by 78.4%, 47.3%, and 54.1% of patients, respectively. EASI 75 was achieved by 62.9%, 88.2%, and 95.5% of patients with Fitzpatrick skin phototype IV (Nx = 35), V (Nx = 17), and VI (Nx = 22), respectively, at Week 24. Most patients (64.4%) with baseline PDCA-Derm™-assessed hyperpigmented areas showed reduced hyperpigmentation at Week 24. Most treatment-emergent adverse events were mild or moderate in severity; none were serious or led to discontinuation. One case of conjunctivitis was reported.
Conclusion: In this first lebrikizumab study in patients with skin of color (Fitzpatrick skin phototype IV, V, and VI) and moderate-to-severe AD, lebrikizumab improved signs and symptoms of AD and confirmed its favorable safety profile.
Trial registration: ClinicalTrials.gov Identifier: NCT05372419 (registered May 5, 2022).
© 2025. The Author(s).
Conflict of interest statement
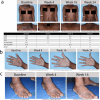
Declarations. Ethics Approval: The ADmirable clinical trial was conducted in accordance with ethical principles outlined in the Declaration of Helsinki, Council for International Organizations of Medical Sciences, and Good Clinical Practice guidelines. The ADmirable trial protocol was approved by the Advarra Institutional Review Board on 17 October 2022 (IRB #: Pro00066938). Additionally, Advarra approved the study at each participating center (Supplement 1, Table S1). All investigation sites received approval from the appropriate authorized institutional review board or ethics committee. The ADmirable study is registered on ClinicalTrials.gov (NCT05372419). Consent to Participate: Informed consent was obtained from all patients before study procedures were initiated. For patients considered to be minors, the written consent of the parent or legal guardian, as well as the assent of the minor, was obtained. Consent to Publish: The authors affirm that human research participants provided informed consent for publication of the images in Figures 3a, 3b, and 3c. Funding and Role of the Funder/Sponsor: This study was funded by Eli Lilly and Company. Eli Lilly and Company had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Almirall, S.A. has licensed the rights to develop and commercialize lebrikizumab for the treatment of dermatology indications, including atopic dermatitis, in Europe. Lilly has exclusive rights for development and commercialization of lebrikizumab in the United States and the rest of the world outside of Europe. Competing Interests: Andrew Alexis has received grant support (funds to institution) from Leo, Amgen, Galderma, Arcutis, Dermavant, Abbvie, Castle Advisory board/Consulting: Leo, Galderma, Pfizer, Sanofi-Regeneron, Genzyme, Dermavant, Beiersdorf, Ortho, L’Oréal, BMS, Bausch health, UCB, Arcutis, Janssen, Allergan, Almirall, Abbvie, Amgen, VisualDx, Eli Lilly and Company, Swiss American, Cutera, Cara, EPI, Incyte, Castle, Apogee, Canfield, Alphyn, Avita Medical, Genentech; has served as a speaker for Regeneron, SANOFI-Genzyme, BMS, L’Oreal, Janssen, and J&J; has received royalties from Springer, Wiley-Blackwell, Wolters Kluwer Health; and has received equipment from Aerolase. Ali Moiin has received reimbursement for travel costs from and conducted clinical trials for Eli Lilly and Company. Jill Waibel has served as a consultant, investigator, and/or on scientific advisory boards for Allergan (Consultant), Amgen (Clinical Trial), ArgenX (Clinical Trial), Bellamia (Advisory Board), Bristol Myers Squibb (Clinical Trial), Candela (Speaker, Consultant, Advisory Board), Cytrellis Biosystems (Advisory Board, Consultant, Clinical Trial), Eli Lilly and Company (Clinical Trial, Speaker), Emblation (Clinical Trial), Galderma (Clinical Trial, Consultant), Horizon (Clinical Trial), Janssen/J&J (Clinical Trial), Lumenis (Advisory Board, Consultant, Speaker), Neuronetics (Clinical Trial), Pfizer (Clinical Trial), P & G (Consultant), RegenX (Clinical Consultant, Clinical Trial, Board of Directors), Sanofi (Clinical Trial), Skinceuticals (Clinical Trial, Consultant, Advisory Board), and Shanghai Biopharma, PWB (Clinical Trial); and has received a VA Merit Grant for Amputated Veterans. Paul Wallace has served as a principal investigator, advisor and/or speaker for Abbvie, Amgen, Arcutis, Biogen, Bristol Myers, Celgene, Centocor, Cynosure, Eli Lilly and Company, Genentech, GlaxoSmithKline, Merck, Novartis, Pfizer, Sanofi-Aventis, and UCB; and has received equipment (loan to institution) from Cynosure. David Cohen reports no financial conflicts of interest. Vivian Laquer conducts research for Abbvie, Acelyrin, Acrotech, Amgen, Argenx, Arcutis, Aslan, Biofrontera, Bristol Meyers Squibb, Cara, Dermavant, Eli Lilly and Company, Galderma, Horizon Therapeutics, Incyte, Janssen, Leo, Novartis, Padagis, Pfizer, Q32, Rapt, Sun, UCB, and Ventyx. Pearl Kwong is a principal investigator for Eli Lilly and Company, Pfizer, Dermavant, Incyte, Arcutis, Galderma, Novartis, Abbvie, CastleCreek Biosciences, Amgen, and UCB; a consultant/advisor for Leo, Galderma, Pfizer, Eli Lilly and Company, Incyte, EPI Health, Novan, Verrica, BMS, Sanofi-Regeneron, UCB, and Ortho; and a speaker for Abbvie, Regeneron Sanofi, Incyte, Verrica, Sun Pharma, Ortho, L’Oréal, EPI, and Arcutis. Amber Reck Atwater is a former employee of Eli Lilly and Company. Jennifer Proper, Evangeline Pierce, Christopher Schuster, Maria Silk, Sreekumar Pillai, and Maria Jose Rueda are employees and shareholders of Eli Lilly and Company. Angela Moore has received research grants or honoraria from Abbvie, Acrotech, Arcutis, Bristol Meyers Squibb, Cara, Eli Lilly and Company, Galderma, Incyte, Janssen J&J, Pfizer, Rapt, and Sanofi-Regeneron. Authors’ Contribution: A. Alexis has contributed to conception of the study and interpretation of the data. A. Moiin has contributed to conception of the work. A. Moore, J. Waibel, and P. Wallace have contributed to acquisition and interpretation of data. D. Cohen and V. Laquer have contributed to acquisition of data. P. Kwong has contributed to interpretation of data. A. Atwater, M. Silk, E. Pierce, S. Pillai, and M.J. Rueda have contributed to study conception, design, analysis, and data interpretation. J. Proper has contributed to study analysis and interpretation. C. Schuster has contributed to data interpretation. All authors contributed to the drafting or critical review of the manuscript and give final approval of the manuscript. Prior Meeting Presentation: Initial baseline characteristics were presented at Maui Derm for Dermatologists, 22 January 2024, Maui, USA. An interim analysis of the results was presented at the American Academy of Dermatology Annual Meeting, 10 March 2024, San Diego, USA. Primary results were presented at the Fall Clinical Dermatology Conference, 24 October 2024, Las Vegas, USA. The 24-week results were presented at the Revolutionizing Atopic Dermatitis (RAD) Conference 2025, June 7, 2025, Nashville, USA. Data Availability: Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the US and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org . Code Availability: Not applicable.
Figures



References
-
- Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396:345–60. 10.1016/S0140-6736(20)31286-1. - PubMed
-
- Quan VL, Erickson T, Daftary K, et al. Atopic dermatitis across shades of skin. Am J Clin Dermatol. 2023;24(5):731–51. 10.1007/s40257-023-00797-1. - PubMed
-
- Silverberg JI. Racial and ethnic disparities in atopic dermatitis. Curr Dermatol Rep. 2015;4(1):44–8. 10.1007/s13671-014-0097-7.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

