MRI-based texture analysis for the evaluation of the response to neoadjuvant chemoimmunotherapy in locally advanced head and neck squamous cell carcinoma
- PMID: 40665208
- PMCID: PMC12261655
- DOI: 10.1186/s12880-025-01806-x
MRI-based texture analysis for the evaluation of the response to neoadjuvant chemoimmunotherapy in locally advanced head and neck squamous cell carcinoma
Abstract
Background: Neoadjuvant chemoimmunotherapy (NCIT) has emerged as a promising approach for patients with locally advanced head and neck squamous cell carcinoma (LA-HNSCC). However, the risk of immune-related adverse events (irAEs) should be taken seriously. And subsequent treatment strategies are determined on the basis of the neoadjuvant effect. Therefore, identifying a robust and effective method to recognize sensitive patients and monitor treatment response is highly important. The study investigated the ability of texture analysis of MRI to predict treatment response after NCIT in patients with LA-HNSCC, and compared it with several clinical indicators.
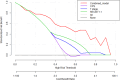
Methods: This retrospective study included 49 LA-HNSCC patients who received NCIT followed by surgery. Texture features were extracted from MR images taken before and after NCIT. Delta features were defined as the percentage change from pre- to posttreatment features. Features that were significantly different between the pathological complete response (pCR) and non pCR groups were selected. Then the features with high diagnostic efficiency and low correlation were subsequently included in logistic regression analysis. Various diagnostic models were constructed via logistic regression, support vector machine (SVM), random forest (RF), and AdaBoost. Several clinical indicators, including tumor stage, combined positive score (CPS) derived from pretreatment lesions, and RECIST 1.1 evaluations by clinicians, were analyzed. ROC analysis and the Delong test were used to assess the performance of various models.
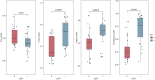
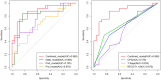
Results: A total of 24 (49.0%) patients achieved pCR, and 25 (51.0%) did not. The Pre_model, Post_model, and Delta_model demonstrated AUCs of 0.678, 0.795, and 0.805, respectively. Compared with the T stage (AUC 0.635), CPS (AUC 0.576), and RECIST1.1 criteria (AUC 0.670) (all p < 0.005), the Combined_model showed better performance, with an AUC of 0.868, a F1-score of 0.824.
Conclusion: Texture analysis based on pre- and posttreatment MR images outperformed the T stage, CPS, and RECIST 1.1 criteria in predicting pathological response following NCIT in patients with LA-HNSCC.
Clinical trial number: Not applicable.
Keywords: Head and neck squamous cell carcinoma; Neoadjuvant chemoimmunotherapy; Pathological complete response; Texture analysis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted according to the tenets of the Declaration of Helsinki. The ethics committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College approved this retrospective study and waived the requirement for informed consent. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Neoadjuvant Chemoimmunotherapy for Resectable Head and Neck Squamous Cell Carcinoma: Systematic Review and Meta-analysis.Ann Surg Oncol. 2025 Jul;32(7):5206-5217. doi: 10.1245/s10434-025-17195-y. Epub 2025 Mar 18. Ann Surg Oncol. 2025. PMID: 40102288
-
Safety and efficacy of neoadjuvant therapy with tislelizumab plus chemotherapy for locally advanced head and neck squamous cell carcinoma: a single-arm, retrospective study.Cancer Immunol Immunother. 2025 Feb 11;74(3):108. doi: 10.1007/s00262-025-03953-0. Cancer Immunol Immunother. 2025. PMID: 39932534 Free PMC article.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Prediction of pathological complete response in locally advanced head and neck squamous cell carcinoma treated with neoadjuvant chemo-immunotherapy using volumetric multisequence MRI histogram analysis.Neuroradiology. 2024 Jun;66(6):919-929. doi: 10.1007/s00234-024-03339-6. Epub 2024 Mar 20. Neuroradiology. 2024. PMID: 38503986 Clinical Trial.
-
Neoadjuvant chemoimmunotherapy for locally advanced squamous cell carcinoma of the head and neck: Systematic review and meta-analysis.Pharmacol Res. 2025 Feb;212:107598. doi: 10.1016/j.phrs.2025.107598. Epub 2025 Jan 11. Pharmacol Res. 2025. PMID: 39805352
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386–96. - PubMed
-
- Braakhuis BJ, Brakenhoff RH, Leemans CR. Treatment choice for locally advanced head and neck cancers on the basis of risk factors: biological risk factors. Ann Oncol. 2012;23(Suppl 10):x173–177. - PubMed
-
- Wise-Draper TM, Gulati S, Palackdharry S, Hinrichs BH, Worden FP, Old MO, Dunlap NE, Kaczmar JM, Patil Y, Riaz MK, et al. Phase II clinical trial of neoadjuvant and adjuvant pembrolizumab in resectable Local-Regionally advanced head and neck squamous cell carcinoma. Clin Cancer Res. 2022;28(7):1345–52. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

