The tumor microbiome in cancer progression: mechanisms and therapeutic potential
- PMID: 40665305
- PMCID: PMC12261620
- DOI: 10.1186/s12943-025-02403-w
The tumor microbiome in cancer progression: mechanisms and therapeutic potential
Abstract
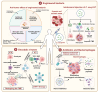
The tumor microbiome (TM) comprises diverse microbial communities, such as bacteria, fungi, and viruses. Recent advancements in microbial sequencing technologies have improved our understanding of the distribution and functional roles of microbes in solid tumors. The TM is formed through several mechanisms, such as direct invasion of mucosal barriers, diffusion from adjacent normal tissues, metastasis of tumor cells, and dissemination via blood and lymphatic circulation. Microbes play a critical role in the tumor microenvironment (TME), and the TM has a heterogeneous composition in different types of cancer. This heterogeneity affects tumor development, progression, and response to treatment. The TM modulates tumor cell physiology and immune responses via several signaling pathways, such as WNT/β-catenin, NF-κB, toll-like receptors (TLRs), ERK, and stimulator of interferon genes (STING). Extensive studies have characterized the role of TM in tumor progression, revealing the importance of genetic abnormalities, epigenetic changes, metabolic regulation, invasion and metastasis, and chronic inflammatory responses. The role of TM in cancer treatment, especially in immunotherapy, has received increasing attention, demonstrating significant regulatory potential. This review provides an in-depth overview of the development of TM detection technologies, explores its potential origins and heterogeneity, and elucidates the mechanisms by which TM contributes to tumorigenesis or tumor suppression. Furthermore, this review explored how TM can be used in cancer treatment, offering a comprehensive perspective on targeted and personalized approaches.
Keywords: Cancer progression; Immune regulation; Microbial metabolites; Therapeutic interventions; Tumor microbiome.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: All of the authors read and approved the fnal manuscript for publication. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Microbiome-mediated immune modulation in tumor microenvironment.Int Rev Cell Mol Biol. 2025;394:107-145. doi: 10.1016/bs.ircmb.2024.12.012. Epub 2025 Feb 17. Int Rev Cell Mol Biol. 2025. PMID: 40623764 Review.
-
Depressive Disorder Affects TME and Hormonal Changes Promoting Tumour Deterioration Development.Immunology. 2025 Aug;175(4):403-418. doi: 10.1111/imm.13933. Epub 2025 May 8. Immunology. 2025. PMID: 40341563 Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Fungi and cancer: unveiling the complex role of fungal infections in tumor biology and therapeutic resistance.Front Cell Infect Microbiol. 2025 Jun 10;15:1596688. doi: 10.3389/fcimb.2025.1596688. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40557321 Free PMC article. Review.
-
Immune evasion in cancer: mechanisms and cutting-edge therapeutic approaches.Signal Transduct Target Ther. 2025 Jul 31;10(1):227. doi: 10.1038/s41392-025-02280-1. Signal Transduct Target Ther. 2025. PMID: 40739089 Free PMC article. Review.
References
-
- Battaglia TW, Mimpen IL, Traets JJH, van Hoeck A, Zeverijn LJ, Geurts BS, et al. A pan-cancer analysis of the Microbiome in metastatic cancer. Cell. 2024;187(9):2324–e3519. - PubMed
-
- Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. 2023;20(7):429–52. - PubMed
-
- Eisenhofer R, Minich JJ, Marotz C, Cooper A, Knight R, Weyrich LS. Contamination in low microbial biomass Microbiome studies: issues and recommendations. Trends Microbiol. 2019;27(2):105–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

