Deep learning-based delineation of whole-body organs at risk empowering adaptive radiotherapy
- PMID: 40665308
- PMCID: PMC12265227
- DOI: 10.1186/s12911-025-03062-z
Deep learning-based delineation of whole-body organs at risk empowering adaptive radiotherapy
Abstract
Background: Accurate delineation of organs at risk (OARs) is crucial for precision radiotherapy. Most previous autosegmentation models were only constructed for single anatomical region without evaluation of dosimetric impact. We aimed to validate the clinical practicability of deep-learning (DL) models for autosegmentation of whole-body OARs with respect to delineation accuracy, clinical acceptance and dosimetric impact.
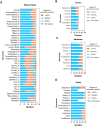
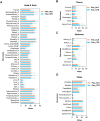
Methods: OARs in various anatomical regions, including the head and neck, thorax, abdomen, and pelvis, were automatedly delineated by DL models (DLD) and compared to manual delineations (MD) by an experienced radiation oncologist (RO). The geometric performance was evaluated using the Dice similarity coefficient (DSC) and average surface distance (ASD). RO A corrected DLD to create delineations approved in clinical practice (CPD). RO B graded the accuracy of DLD to assess clinical acceptance. The dosimetric impact was determined by assessing the difference in dosimetric parameters for each OAR in the DLD-based radiotherapy plan (Plan_DLD) and the CPD-based radiotherapy plan (Plan_CPD).
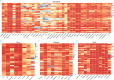
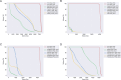
Results: The automatic delineation model has a high OAR delineation accuracy, and the median DSCs can reach 0.841 (IQR, 0.791-0.867) in the head and neck OAR, 0.903 (IQR, 0.777-0.932) in thoracic OAR, 0.847 (IQR, 0.834-0.931) in abdominal OAR, 0.916 (IQR, 0.906-0.964) in pelvic OAR. The majority of DL-generated OARs were graded as clinically acceptable with no editing or little editing needed. No significant differences in dosimetric parameters were found by comparing Plan_DLD with Plan_CPD.
Conclusions: For OARs of whole bodily regions, DL-based segmentation is fast; DL models perform sufficiently well for clinical practice with respect to delineation accuracy, clinical accepatance and dosimetric impact.
Keywords: Auto-delineation; Deep learning; Whole-body organs at risk.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study protocol was reviewed and approved by Sun Yat-sen University Cancer Center Ethics Committee (B2023-173-01). The study adhered to the principles of the Declaration of Helsinki. Consent to participate: Due to the retrospective nature of the study and only deidentified data included, the Sun Yat-sen University Cancer Center Ethics Committee waived the need for written informed consent. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Lim JY, Leech M. Use of auto-segmentation in the delineation of target volumes and organs at risk in head and neck. Acta Oncol. 2016;55(7):799–806. - PubMed
-
- Verhaart RF, et al. CT-based patient modeling for head and neck hyperthermia treatment planning: manual versus automatic normal-tissue-segmentation. Radiother Oncol. 2014;111(1):158–63. - PubMed
-
- Vinod SK, et al. Uncertainties in volume delineation in radiation oncology: a systematic review and recommendations for future studies. Radiother Oncol. 2016;121(2):169–79. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

