p38 mitogen-activated protein kinase drives senescence in CD4+ T lymphocytes and increases their pathological potential
- PMID: 40665343
- PMCID: PMC12261694
- DOI: 10.1186/s12979-025-00526-8
p38 mitogen-activated protein kinase drives senescence in CD4+ T lymphocytes and increases their pathological potential
Abstract
Background: In several diseases, senescent T lymphocytes increase in number and release a senescence-associated secretory phenotype (SASP) with inflammatory and osteoclastogenic potential, favoring inflammation and bone loss. It is well known that the activation of p38 mitogen-activated protein kinase (p38 MAPK) orchestrates senescence in CD8+ T lymphocytes. However, p38 MAPK contribution to CD4+ T lymphocyte senescence remains less comprehensively characterized and warrants further investigation. This study investigates the contribution of p38 MAPK to senescence in CD4+ T lymphocytes, focusing on mitochondrial dysfunction and SASP production to elucidate their pathological potential.
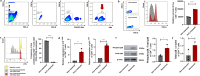
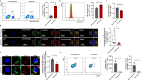
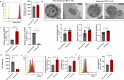
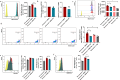
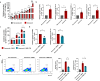
Results: Splenic CD4+ T lymphocytes isolated from wild-type C57BL/6 mice were subjected to subcytotoxic oxidative stress by H2O2 exposure to generate stress-induced premature senescence. H2O2-exposed CD4+ T lymphocytes exhibited hallmark features of senescence, including increased cell size, reduced cell proliferation, and upregulation of the cell cycle regulators p16Ink4a and p21Cip1. Additionally, these cells displayed defective mitophagy, accumulation of dysfunctional mitochondria, and a SASP enriched in Th17-associated cytokines. In senescence-induced CD4+ T lymphocytes, an increase in the expression of phospho-p38 MAPK was also detected. The senescence changes were reversed when p38 MAPK was blocked using the specific inhibitor BIRB-796. In particular, neutralizing p38 MAPK reduced mitochondrial dysfunction and Th17-type SASP production, demonstrating its critical role in driving these senescent traits in CD4+ T lymphocytes. These findings ratify the involvement of p38 MAPK as a central regulator of CD4+ T lymphocyte senescence, particularly concerning the accumulation of dysfunctional mitochondria and pro-inflammatory SASP production.
Conclusions: This study provides critical insights into immune aging mechanisms in CD4+ T lymphocytes and underscores the therapeutic potential of targeting p38 MAPK to mitigate senescence-driven inflammatory diseases.
Keywords: CD4-positive T-Lymphocyte; Cellular senescence; Mitophagy; SASP; p38 MAPK.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: The study protocol was approved by the Institutional Committee for the Care and Use of Animals (CICUA), Protocol #ODO-UCH 23656. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Downregulation of LATS1/2 Drives Endothelial Senescence-Associated Stemness (SAS) and Atherothrombotic Lesion Formation.bioRxiv [Preprint]. 2025 Jun 21:2025.06.19.660635. doi: 10.1101/2025.06.19.660635. bioRxiv. 2025. PMID: 40667385 Free PMC article. Preprint.
-
Chk2 deletion rescues bone loss and cellular senescence induced by Bmi1 deficiency via regulation of Cyp1a1.J Orthop Translat. 2025 May 10;52:360-375. doi: 10.1016/j.jot.2025.04.014. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40698069 Free PMC article.
-
Is T-cell senescence associated with inflammatory arthritis and disease burden? A systematic review.Semin Arthritis Rheum. 2025 Aug;73:152757. doi: 10.1016/j.semarthrit.2025.152757. Epub 2025 May 14. Semin Arthritis Rheum. 2025. PMID: 40393379 Review.
-
Therapeutic effects of PDGF-AB/BB against cellular senescence in human intervertebral disc.Elife. 2025 Jul 16;13:RP103073. doi: 10.7554/eLife.103073. Elife. 2025. PMID: 40668091 Free PMC article.
-
p38 MAPK signaling in chronic obstructive pulmonary disease pathogenesis and inhibitor therapeutics.Cell Commun Signal. 2023 Nov 2;21(1):314. doi: 10.1186/s12964-023-01337-4. Cell Commun Signal. 2023. PMID: 37919729 Free PMC article.
References
-
- Hernandez-Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018;28(6):436–53. - PubMed
-
- Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: A culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2024;10(3):119–24. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

