Liensinine reshapes the immune microenvironment and enhances immunotherapy by reprogramming metabolism through the AMPK-HIF-1α axis in hepatocellular carcinoma
- PMID: 40665352
- PMCID: PMC12261578
- DOI: 10.1186/s13046-025-03477-6
Liensinine reshapes the immune microenvironment and enhances immunotherapy by reprogramming metabolism through the AMPK-HIF-1α axis in hepatocellular carcinoma
Abstract
Background: Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality, with limited treatment options in advanced stages. Liensinine, a natural alkaloid derived from Nelumbo nucifera, has shown promise as an anticancer agent. However, its underlying mechanisms, particularly in modulating tumor metabolism and immune responses, remain poorly understood. This study aimed to investigate the antitumor effects of Liensinine in HCC, focusing on its ability to modulate metabolic pathways, immune responses, and the tumor microenvironment.
Methods: HCC cell lines (HUH7 and Hep1-6) were treated with Liensinine in vitro to assess cell viability, migration, proliferation, and apoptosis. Metabolic reprogramming was analyzed through RNA sequencing, Seahorse metabolic assays, and glucose/lactate measurements. The effects on immune cells were studied by treating THP-1 macrophages and peripheral blood mononuclear cells (PBMCs) with conditioned media from Liensinine-treated cells. In vivo, subcutaneous xenograft and orthotopic liver cancer models were used to evaluate the therapeutic efficacy of Liensinine combination with radiotherapy and immunotherapy.
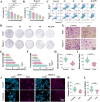
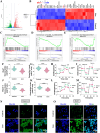
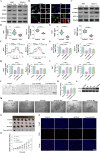
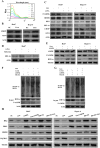
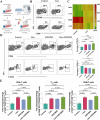
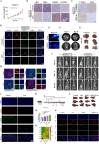
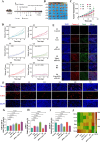
Results: Liensinine inhibited HCC cell viability, migration, and proliferation, promoting apoptosis and shifting metabolism from glycolysis to oxidative phosphorylation. This metabolic reprogramming was linked to the activation of the AMPK-HIF-1α axis and increased ROS production. Furthermore, Liensinine induced Endoplasmic reticulum (ER) stress, as evidenced by elevated levels of CHOP and ATF4, which contributed to AMPK activation and suppression of HIF-1α. Liensinine reduced PD-L1 expression, enhanced M1 macrophage polarization, and promoted CD8 + T cell infiltration into tumors. In vivo, Liensinine significantly suppressed tumor growth, reduced vascular density, and reshaped the immune microenvironment by promoting M1 macrophage polarization. Combination therapy with Liensinine, radiotherapy, and immunotherapy resulted in synergistic effects, including enhanced tumor cell apoptosis, increased immune cell infiltration, and improved therapeutic efficacy.
Conclusion: Liensinine exerts potent antitumor effects in HCC by reprogramming tumor metabolism, inducing ER stress, enhancing immune responses, and modulating the TME. The combination of Liensinine with immunotherapy and radiotherapy significantly improves therapeutic efficacy, suggesting its potential as a novel treatment strategy for HCC.
Keywords: AMPK; Angiogenesis; HCC; Hif-1α; Immunotherapy; Liensinine; Macrophage polarization; Metabolic reprogramming.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approved: Animal studies and clinical samples were conducted in accordance with the Declaration of Helsinki and by the Ethics Committee of Xiangya Third Hospital, Central South University (22480). Competing interests: The authors declare no competing interests.
Figures
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Li X, Ramadori P, Pfister D, Seehawer M, Zender L, Heikenwalder M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer. 2021;21(9):541–57. - PubMed
-
- Yang H, Cheng J, Zhuang H, Xu H, Wang Y, Zhang T, et al. Pharmacogenomic profiling of intra-tumor heterogeneity using a large organoid biobank of liver cancer. Cancer Cell. 2024;42(4):535–51. e8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

