Respiratory syncytial virus (RSV) enhances translation of virus-resembling AU-rich host transcripts
- PMID: 40665364
- PMCID: PMC12265200
- DOI: 10.1186/s12985-025-02838-z
Respiratory syncytial virus (RSV) enhances translation of virus-resembling AU-rich host transcripts
Abstract
Background: Viruses strongly rely on the host's translational machinery to produce viral proteins required for replication. However, it is unknown how viruses that do not globally inhibit cap-dependent translation compete with abundant host transcripts for ribosomes. Viral infection often triggers eukaryotic initiator factor 2α (eIF2α) phosphorylation, leading to global 5'-cap-dependent translation inhibition. Respiratory syncytial virus (RSV) encodes mRNAs mimicking 5'-cap structures of host mRNAs and thus inhibition of cap-dependent translation initiation would likely also reduce viral translation.
Methods: RSV-infected HEp-2 and A549 cells were analyzed to determine translation levels using western blotting, indirect immunofluorescent staining and polysome profiling. Transcriptome-wide translation efficiencies of virus-infected cells were compared against mock-infected cells using high-throughput sequencing of poly(A)-tail enriched total mRNA and transcripts associated with heavy polysomes.
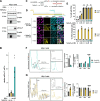
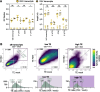
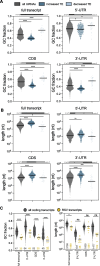
Results: We confirmed that RSV limits widespread translation initiation inhibition and unexpectedly found that the fraction of ribosomes within polysomes increases during infection, indicating higher ribosome loading on mRNAs during infection. High-throughput sequencing revealed that virus-resembling, AU-rich host transcripts become more efficient at ribosome recruitment. Using a previously published dataset, we observe similar trends in another negative-sense single-stranded RNA virus, vesicular stomatitis virus (VSV).
Conclusions: These findings revealed that RSV changes the translational landscape by enhancing translation of virus-resembling AU-rich host transcripts rather than inhibiting host translation.
Keywords: AU-rich transcripts; High-throughput sequencing; Polysome profiling; RSV; Translation efficiency; VSV.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Respiratory Syncytial Virus (RSV) optimizes the translational landscape during infection.bioRxiv [Preprint]. 2024 Aug 3:2024.08.02.606199. doi: 10.1101/2024.08.02.606199. bioRxiv. 2024. PMID: 39131278 Free PMC article. Preprint.
-
Host cellular transcriptional response to respiratory syncytial virus infection in HEp-2 cells: insights from cDNA microarray and quantitative PCR analyses.Front Cell Infect Microbiol. 2025 Jun 24;15:1613386. doi: 10.3389/fcimb.2025.1613386. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40630640 Free PMC article.
-
CypA inhibits respiratory syncytial virus (RSV) replication by suppressing glycolysis through the downregulation of PKM2 expression.J Virol. 2025 Jul 22;99(7):e0007425. doi: 10.1128/jvi.00074-25. Epub 2025 Jun 24. J Virol. 2025. PMID: 40552816 Free PMC article.
-
Host immune response to respiratory syncytial virus infection and its contribution to protection and susceptibility in adults: a systematic literature review.Expert Rev Clin Immunol. 2025 Jun;21(6):745-760. doi: 10.1080/1744666X.2025.2494658. Epub 2025 May 2. Expert Rev Clin Immunol. 2025. PMID: 40278893 Review.
-
Immunoglobulin treatment for hospitalised infants and young children with respiratory syncytial virus infection.Cochrane Database Syst Rev. 2023 Oct 23;10(10):CD009417. doi: 10.1002/14651858.CD009417.pub3. Cochrane Database Syst Rev. 2023. PMID: 37870128 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

