Combinatorial targeting of NMDARs and 5-HT4Rs exerts beneficial effects in a mouse model of Alzheimer's disease
- PMID: 40665449
- PMCID: PMC12261665
- DOI: 10.1186/s13195-025-01804-9
Combinatorial targeting of NMDARs and 5-HT4Rs exerts beneficial effects in a mouse model of Alzheimer's disease
Abstract
Background: Alzheimer's disease (AD) is the leading cause of dementia. There are limited approved medications that delay cognitive decline or lessen neuropsychiatric symptoms. Numerous clinical trials for AD using a single drug administration have failed to meet therapeutic endpoints, which is most likely due to the complexity of AD. A multimodal therapeutic intervention is more likely to improve symptoms by targeting multiple targets implicated in AD. Here, we investigated if targeting both N-Methyl-D-aspartic acid receptors (NMDARs) and serotonin type 4 receptors (5-HT4R) may have beneficial effects in a mouse model of AD, as they have separately been shown to improve cognition and/or mood.
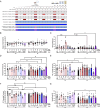
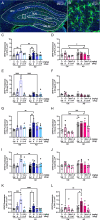
Methods: Male and female control (Ctrl) or APP/PS1 mice were administered single, intermittent, or chronic administration of 1) saline; 2) (R,S)-ketamine, an NMDAR antagonist; 3) prucalopride, a 5-HT4R agonist; or 4) (R,S)-ketamine + prucalopride to simultaneously target co-morbid neuropsychiatric and cognitive deficits. Behavioral assays were then administered to measure cognition, perseverative behavior, hyponeophagia, and/or sleep. Brains were processed for glial fibrillary acidic protein (GFAP) immunohistochemistry.
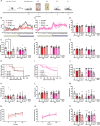
Results: Single and chronic administration of (R,S)-ketamine + prucalopride administration improved cognitive decline by increasing memory retrieval in a contextual fear conditioning (CFC) paradigm in APP/PS1 mice. Drug efficacy was less effective in females than in males and was age dependent. Hippocampal GFAP immunoreactivity was decreased by chronic (R,S)-ketamine + prucalopride treatment in females.
Conclusions: Our results indicate that combined administration of (R,S)-ketamine + prucalopride is a novel multimodal therapeutic strategy to treat cognitive decline in AD. Future work will further characterize these interactions with the goal of clinical development.
Keywords: Adjunctive treatment; Ketamine; Neurodegeneration; Neuroinflammation; Prucalopride.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All procedures were conducted in accordance with the National Institutes of Health (NIH) regulations and by the Institutional Animal Care and Use Committees (IACUCs) of Columbia University Irving Medical Center (CUIMC) and the Research Foundation for Mental Hygiene, Inc. (RFMH) at the New York State Psychiatric Institute (NYSPI). Competing interests: BKC, HCH, and CAD are named on provisional patent applications for the prophylactic use of (R,S)-ketamine, 5-HT4R agonists, and other compounds against stress-related psychiatric disorders and Alzheimer’s disease. SJ is employed by Silo Pharma, and EW is founder and Chief Executive Officer of Silo Pharma. AW, LCM, AY, MJW, and RL have no conflicts of interest to disclose.
Figures


Similar articles
-
Chronic, combinatorial targeting of NMDARs and 5-HT4Rs exerts extended behavioral effects against stress-induced perseverative behavior and hyponeophagia.Neuropsychopharmacology. 2025 Jul;50(8):1210-1223. doi: 10.1038/s41386-025-02107-1. Epub 2025 Apr 22. Neuropsychopharmacology. 2025. PMID: 40263416
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Selegiline for Alzheimer's disease.Cochrane Database Syst Rev. 2003;(1):CD000442. doi: 10.1002/14651858.CD000442. Cochrane Database Syst Rev. 2003. PMID: 12535396
-
Galantamine for Alzheimer's disease.Cochrane Database Syst Rev. 2002;(3):CD001747. doi: 10.1002/14651858.CD001747. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2004 Oct 18;(4):CD001747. doi: 10.1002/14651858.CD001747.pub2. PMID: 12137632 Updated.
-
Galantamine for Alzheimer's disease.Cochrane Database Syst Rev. 2001;(4):CD001747. doi: 10.1002/14651858.CD001747. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2002;(3):CD001747. doi: 10.1002/14651858.CD001747. PMID: 11687119 Updated.
References
-
- NIA-funded active Alzheimer’s and related dementias clinical trials and studies. Natl Inst Aging. Available from: https://www.nia.nih.gov/research/ongoing-AD-trials. Cited 2025 Feb 13.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

