Pittsburgh plasma p-tau217: Classification accuracies for autosomal dominant and sporadic Alzheimer's disease in the community
- PMID: 40665482
- PMCID: PMC12263344
- DOI: 10.1002/alz.70409
Pittsburgh plasma p-tau217: Classification accuracies for autosomal dominant and sporadic Alzheimer's disease in the community
Abstract
Introduction: Most available phosphorylated tau (p-tau)217 immunoassays have similar performance. It is unclear if this is due to the use of the same antibody (the "ALZpath antibody"). We established and evaluated a novel p-tau217 assay that uses an alternative antibody and benchmarked the results against ALZpath-p-tau217.
Methods: After development and analytical validation of the University of Pittsburgh ("Pitt-p-tau217") method, clinical verification was performed in three independent cohorts (n = 363).
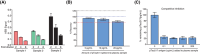
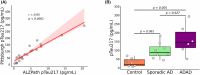
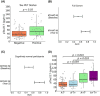
Results: Pitt-p-tau217 demonstrated high between-run stability, linearity, and specificity. Clinically, Pitt-p-tau217 differentiated neuropathologically confirmed PSEN1 mutation carriers from controls with area under the curve (AUC) = 0.94, and amyloid beta (Aβ) positron emission tomography (PET)-positive from Aβ PET-negative cognitively normal older adults with AUC up to 0.84, equivalent to ALZpath-p-tau217 results. Both Pitt-p-tau217 and ALZpath-p-tau217 were slightly elevated in tau PET-positive versus tau PET-negative participants. Between-assay correlations were up to 0.93.
Discussion: The new Pitt-p-tau217 assay exhibits high and reproducible classification accuracies for identifying individuals with biological evidence of Alzheimer's disease, equivalent to the widely used ALZpath-p-tau217.
Highlights: We designed and developed an alternative assay to quantify plasma phosphorylated tau (p-tau)217, aiming to enhance accuracy and enable early detection of Alzheimer's disease (AD). Comprehensive analytical and clinical validation demonstrated that the new p-tau217 assay is a valuable and affordable resource for investigating AD pathophysiology. The new p-tau217 assay showed similar performance to the established ALZpath assay in staging and monitoring early AD.
Keywords: Alzheimer's disease; autosomal dominant Alzheimer's disease; blood‐based biomarkers; phosphorylated tau217; population‐based studies.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
T.K.K. has consulted for Quanterix Corporation, SpearBio Inc., and Neurogen Biomarking LLC; has served on advisory board for Neurogen Biomarking LLC; and has received in‐kind research support from Janssen Research Laboratories and Alamar Biosciences, outside the submitted work. He has received honoraria for speaker/grant review engagements from the NIH, UPENN, UW‐Madison, Advent Health, Brain Health conference, Barcelona‐Pittsburgh conference, the International Neuropsychological Society, the Icahn School of Medicine at Mount Sinai, and CQDM Canada, all outside of the submitted work. T.K.K. is an inventor on several patents and provisional patents regarding biofluid biomarker methods, targets, and reagents/compositions, that may generate income for the institution and/or self should they be licensed and/or transferred to another organization. X.Z., E.E.A. and M.D.I. are inventors on University of Pittsburgh provisional patents together with T.K.K. The other authors report no conflict of interest. Author disclosures are available in the supporting information.
Figures




Update of
-
Pittsburgh plasma p-tau217: classification accuracies for autosomal dominant and sporadic Alzheimer's disease in the community.medRxiv [Preprint]. 2025 May 6:2025.05.03.25326526. doi: 10.1101/2025.05.03.25326526. medRxiv. 2025. Update in: Alzheimers Dement. 2025 Jul;21(7):e70409. doi: 10.1002/alz.70409. PMID: 40385398 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- R01 AG075336/AG/NIA NIH HHS/United States
- U01 NS131740/NS/NINDS NIH HHS/United States
- R01 AG083874/AG/NIA NIH HHS/United States
- U24AG082930/AG/NIA NIH HHS/United States
- R01 AG072641/AG/NIA NIH HHS/United States
- HT94252320064/DoD
- U01 NS141777/NS/NINDS NIH HHS/United States
- P30 AG066468/AG/NIA NIH HHS/United States
- R01 AG073267/AG/NIA NIH HHS/United States
- DAF2255207/Aging Mind Foundation
- R01 AG025516/AG/NIA NIH HHS/United States
- R37 AG023651/AG/NIA NIH HHS/United States
- U24 AG082930/AG/NIA NIH HHS/United States
- R01 MH108509/MH/NIMH NIH HHS/United States
- R01 AG083156/AG/NIA NIH HHS/United States
- RF1 AG077474/AG/NIA NIH HHS/United States
- P01 AG025204/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

