IscR-tmRNA regulatory axis plays a key role in multiple stress response and pathogenicity in Aeromonas veronii
- PMID: 40665627
- PMCID: PMC12269673
- DOI: 10.1080/21505594.2025.2530659
IscR-tmRNA regulatory axis plays a key role in multiple stress response and pathogenicity in Aeromonas veronii
Abstract
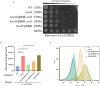
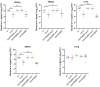
Bacterial pathogens intricately modulate their response to a variety of stress and the virulence, particularly in light of the dynamic conditions both in natural habitat and within host organisms. Transfer-messenger RNA (tmRNA), which plays an important role in pathogenicity due to its major function in the trans-translation system for ribosome rescue, has been proved as a stress response molecule. Herein, our results indicate that the global regulator IscR acts as a crucial activator responsible for the expression of tmRNA in Aeromonas veronii, a bacterial pathogen posing significant challenges to both aquatic industry and public health. Bacterial one-hybrid and electrophoretic mobility shift assays (EMSA) confirm the direct binding of IscR to the promoter region of the ssrA gene which encodes tmRNA. Moreover, our phenotypic characterizations illustrate that the complementation of tmRNA can rescue the defects of iscR deletion in response to adverse stress, including nutrient deprivation, elevated temperatures, β-lactam antibiotics, and oxidative stress, as well as in establishing the pathogenicity characterized by motility, aggregation, adhesion, cytotoxicity, bacterial competition, and colonization in mice. Our findings offer insights into a potential model for strengthening bacterial survival in external environments, and provide an initial glimpse into the intricate interplay between the functional roles of IscR and tmRNA in the pathogenicity through the IscR-tmRNA regulatory axis.
Keywords: Aeromonas veronii; IscR; pathogenicity; stress response; tmRNA; virulence.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



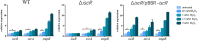



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
